Chemistry (Grades 9–12)
Subtest 1 Sample Items
Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
Expand All | Collapse All
Question 1
1. The following experiment has been designed to determine the effect of temperature on the rate of corrosion of an iron nail.
| Control |
Experimental Conditions |
Nail at 22° degrees C cut
into 2 pieces |
Intact nail
at 5° degrees C |
Intact nail
at 35° degrees C |
Intact nail
at 45° degrees C |
Three 2.5 g iron nails were used for the control condition and for each experimental condition. Individual nails were placed into open test tubes containing 10
mL of H2O and they were maintained at the specified temperatures for 48 hours. After the 48-hour incubation period, the presence of corrosion was determined through the use of a chemical indicator. Which of the following best describes the flaw in this experimental design?
- Temperature has little effect on reaction rate.
- The experiment lacks a valid control for the experimental conditions.
- Iron is a poor choice of metal for this experiment.
- The experimental conditions are too difficult to maintain for 48 hours.
Answer to question 1
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0001) An effective control is an important part of good experimental design. The control should be identical to the experimental condition in every way except for the variable being studied. This type of control helps a researcher make valid conclusions about the effect of the experimental condition being tested. In the described experiment, the control condition differs from the experimental conditions in two ways—temperature and surface area. The introduction of a second variable in the control condition complicates the analysis and interpretation of the results. An appropriate control for this experiment would have been an intact nail at 22°C.
Correct Response: B. (Objective 0001) An effective control is an important part of good experimental design. The control should be identical to the experimental condition in every way except for the variable being studied. This type of control helps a researcher make valid conclusions about the effect of the experimental condition being tested. In the described experiment, the control condition differs from the experimental conditions in two ways—temperature and surface area. The introduction of a second variable in the control condition complicates the analysis and interpretation of the results. An appropriate control for this experiment would have been an intact nail at twenty two degrees celsius.
Question 2
2. The table below shows data for the solubility of a gas in water over a range of temperatures.
| Temperature (° degrees C) |
Gas Solubility (mM) |
| 0 |
2.0 |
| 10 |
1.7 |
| 20 |
1.5 |
| 30 |
1.3 |
| 40 |
1.1 |
| 50 |
1.0 |
A chemist wants to use these data to determine the solubility of the gas at 25° degrees C. Which of the following methods of presenting data would be most helpful in making this determination?
- bar graph
- stem-and-leaf plot
- line graph
- frequency diagram
Answer to question 2
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0001) A line graph is the best choice for presenting the solubility data. This method will assist in identifying any trends in gas solubility that exist as a function of water temperature. Plotting the experimental data as a line graph will allow the chemist to make a reasonable determination of the solubility of the gas at 25°C.
Correct Response: C. (Objective 0001) A line graph is the best choice for presenting the solubility data. This method will assist in identifying any trends in gas solubility that exist as a function of water temperature. Plotting the experimental data as a line graph will allow the chemist to make a reasonable determination of the solubility of the gas at twenty five degress celsius.
Question 3
3. The instructions for a titration experiment are shown below.

Materials: buret, clamp, ring stand, two hundred and fifty m upper L beaker, p upper H probe, p upper H meter, glass stirring rod, 1 M Upper N a Upper O upper H, Upper C upper H sub three Upper C Upper O Upper O upper H solution open parenthesis concentration unknown close parenthesis.
Method. bullet prepare a zero point one M Upper N a Upper O upper H solution from the stock solution bullet set up glassware including p upper H probe. bullet place fifty m upper L of upper C upper H sub three upper C upper O upper O upper H into beaker. bullet record the p upper H of the upper C upper H sub three upper C upper O upper O upper H solution. bullet fill the buret with zero point one m upper N a upper O upper H and record the volume. bullet slowly add zero point one m upper N a upper O upper H to beaker bullet stir gently and record the p upper H. bullet continue beyond the equivalence point safety notes. bullet protective eyewear and laboratory aprons must be worn at all times. bullet acid and bases must be disposed of properly
Which of the following represents the greatest potential safety hazard associated with this experiment?
- the process of preparing the 0.1 M NaOH solution
- the effect of prolonged exposure to CH3COOH
- the product formed by combining the acid and the base
- the reactivity of the acid and the base with glassware
Answer to question 3
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0001) Preparing the 0.1 M NaOH solution requires dispensing a volume of the highly corrosive concentrated stock solution. Proper protocols need to be followed to avoid personal injury and spills.
Correct Response: A. (Objective 0001) Preparing the zero point one mole of upper n a upper o upper h solution requires dispensing a volume of the highly corrosive concentrated stock solution. Proper protocols need to be followed to avoid personal injury and spills.
Question 4
4. Which of the components in the voltaic cell shown maintains the electrical neutrality of the two solutions?
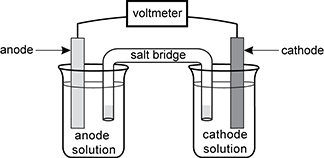
There is an anode in the first beaker connected to a voltmeter that is connected to a cathode in the second beaker. There is also a salt bridge with each end in the beakers connecting the two solutions
- anode
- voltmeter
- cathode
- salt bridge
Answer to question 4
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0001) As the processes of oxidation and reduction proceed, there is a change in the electrical charge of the solutions in the anode and cathode compartments. In order for the electrical circuit to continue to function, the two solutions must remain electrically neutral. This is accomplished by the salt bridge, which contains an inert electrolyte solution. As the reaction proceeds, ions in this electrolyte solution move into the two compartments and neutralize the accumulating charge.
Correct Response: D. (Objective 0001) As the processes of oxidation and reduction proceed, there is a change in the electrical charge of the solutions in the anode and cathode compartments. In order for the electrical circuit to continue to function, the two solutions must remain electrically neutral. This is accomplished by the salt bridge, which contains an inert electrolyte solution. As the reaction proceeds, ions in this electrolyte solution move into the two compartments and neutralize the accumulating charge.
Question 5
5. Use the passage below to answer the question that follows.
In order to determine the SO2(g) content of an air sample, a researcher first converted the SO2(g) into sulfuric acid by bubbling the air sample through an aqueous solution of hydrogen peroxide. The researcher then titrated a standard NaOH solution against a 2.50 mL sample of the prepared acid solution.
In order to determine the capital S O subscript 2 grams content of an air sample, a researcher first converted the capital S capital O subscript 2 grams into sulfuric acid by bubbling the air sample through an aqueous solution of hydrogen peroxide. The researcher then titrated a standard capital N lowercase a capital O H solution against a 2.50 milliliter sample of the prepared acid solution.
Which of the following pieces of glassware is most appropriate for measuring the volume of the sulfuric acid used?
- beaker
- Erlenmeyer flask
- pipette
- graduated cylinder
Answer to question 5
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0001) For the experiment described, the amount of sample used is 2.50
mL. Therefore, the glassware used must measure precisely to the hundredth of a milliliter. A pipette is used to measure with higher precision than the other types of glassware listed; therefore, the pipette would be the best tool for measuring the volume of the sulfuric acid.
Correct Response: C. (Objective 0001) For the experiment described, the amount of sample used is 2.50
mL. Therefore, the glassware used must measure precisely to the hundredth of a milliliter. A pipette is used to measure with higher precision than the other types of glassware listed; therefore, the pipette would be the best tool for measuring the volume of the sulfuric acid.
Question 6
6. Which of the following mathematical equations would be most helpful to a chemist trying to determine the age of a bone found at an archaeological site?
- ln(A/A0) = –kt The natural log of the quantity A divided by A subscript naught equals negative k t
- y = mx + b y equals m x plus b
- ΔG = ΔH –TΔS delta G equals delta H minus T delta S
- x =
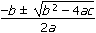 x equals a fraction. The numerator is negative b plus or minus the square root of the quantity b squared minus 4 a c. The denominator is 2 a.
x equals a fraction. The numerator is negative b plus or minus the square root of the quantity b squared minus 4 a c. The denominator is 2 a.
Answer to question 6
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0002) In order to determine the age of a bone found at an archaeological site, the chemist would use a technique called radiometric dating. For organic materials, such as bones, this technique involves determining the amount of carbon-14 present. Carbon-14 is an isotope of carbon-12 that is produced in the upper atmosphere and is incorporated into the tissues of a living organism throughout the course of its life. After the organism dies, the amount of carbon-14 decreases. Because the nuclear decay process is a reaction that follows first order kinetics, the initial rate of decay (A0) and the rate of decay at a specific point in time (A) can be used to calculate how much time has passed using the equation ln(A/A0) = –kt, where k is a rate constant based on the 5,730 year half-life of carbon-14.
Correct Response: A. (Objective 0002) In order to determine the age of a bone found at an archaeological site, the chemist would use a technique called radiometric dating. For organic materials, such as bones, this technique involves determining the amount of carbon-14 present. Carbon-14 is an isotope of carbon-12 that is produced in the upper atmosphere and is incorporated into the tissues of a living organism throughout the course of its life. After the organism dies, the amount of carbon-14 decreases. Because the nuclear decay process is a reaction that follows first order kinetics, the initial rate of decay (A subscript 0) and the rate of decay at a specific point in time (A) can be used to calculate how much time has passed using the equation natural log of A divided by A subscript 0 equals negative K times T, where k is a rate constant based on the 5,730 year half-life of carbon-14.
Question 7
7. Which of the following types of chemistry problems is most likely to involve the use of the quadratic formula?
- calculating the pH of a weak acid with a known molarity and Ka
- determining the enthalpy of a reaction from bond enthalpy values
- calculating the number of moles in a given volume of a gas
- determining the spontaneity of a reaction using Gibbs free energy
Answer to question 7
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0002) Calculating the pH of a weak acid involves expressing the equilibrium concentrations of the starting acid and its ions in terms of the variable x (the change in concentration). These concentration terms can then be substituted into the equilibrium constant expression, which can then be expressed as a quadratic equation (ax2 + bx + c = 0). The quadratic formula,
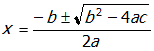 , is used to solve this type of equation. While the quadratic formula can be used to solve all problems of this type, there are cases in which it is not necessary to use it. For example, if a weak acid has a very small acid ionization value, its concentration at equilibrium will not be significantly different from the starting concentration. If this is the case, the equilibrium constant expression can be simplified into a form that does not require using the quadratic formula.
Correct Response: A. (Objective 0002) Calculating the pH of a weak acid involves expressing the equilibrium concentrations of the starting acid and its ions in terms of the variable x (the change in concentration). These concentration terms can then be substituted into the equilibrium constant expression, which can then be expressed as a quadratic equation open parenthesis a x squared plus b x plus c equals zero close parenthesis. The quadratic formula, x equals start fraction numerator negative b plus or minus start square root b squared minus four a c end root denominator two a end fraction, is used to solve this type of equation. While the quadratic formula can be used to solve all problems of this type, there are cases in which it is not necessary to use it. For example, if a weak acid has a very small acid ionization value, its concentration at equilibrium will not be significantly different from the starting concentration. If this is the case, the equilibrium constant expression can be simplified into a form that does not require using the quadratic formula.
, is used to solve this type of equation. While the quadratic formula can be used to solve all problems of this type, there are cases in which it is not necessary to use it. For example, if a weak acid has a very small acid ionization value, its concentration at equilibrium will not be significantly different from the starting concentration. If this is the case, the equilibrium constant expression can be simplified into a form that does not require using the quadratic formula.
Correct Response: A. (Objective 0002) Calculating the pH of a weak acid involves expressing the equilibrium concentrations of the starting acid and its ions in terms of the variable x (the change in concentration). These concentration terms can then be substituted into the equilibrium constant expression, which can then be expressed as a quadratic equation open parenthesis a x squared plus b x plus c equals zero close parenthesis. The quadratic formula, x equals start fraction numerator negative b plus or minus start square root b squared minus four a c end root denominator two a end fraction, is used to solve this type of equation. While the quadratic formula can be used to solve all problems of this type, there are cases in which it is not necessary to use it. For example, if a weak acid has a very small acid ionization value, its concentration at equilibrium will not be significantly different from the starting concentration. If this is the case, the equilibrium constant expression can be simplified into a form that does not require using the quadratic formula.
Question 8
8. In a laboratory exercise, students were asked to determine the boiling point of an unknown liquid. Each lab group performed the exercise multiple times and then calculated the mean boiling point temperature and the standard deviation of the results. Their results are shown in the table below.
|
Group 1 |
Group 2 |
Group 3 |
Group 4 |
Mean
Boiling
Point (°C) |
79.8 |
81.7 |
82.3 |
79.4 |
Standard
Deviation |
0.8 |
2.5 |
1.1 |
0.3 |
According to these results, which lab group's boiling point data showed the least amount of variation across multiple trials?
- Group 1
- Group 2
- Group 3
- Group 4
Answer to question 8
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0002) Standard deviation is a measure of the variation within a set of data. A low standard deviation value indicates less variation within the data set. Since the standard deviation of Group 4's data is less than that of the other groups, this lab group's boiling point data were the most consistent across multiple trials.
Correct Response: D. (Objective 0002) Standard deviation is a measure of the variation within a set of data. A low standard deviation value indicates less variation within the data set. Since the standard deviation of Group 4's data is less than that of the other groups, this lab group's boiling point data were the most consistent across multiple trials.
Question 9
9. A chemistry class has completed a lab exercise on the effect of temperature on reaction rate. Which of the following next steps would aid them most in understanding their results?
- reviewing the concepts of heat and temperature
- comparing technical difficulties experienced during the experiment
- viewing a computer simulation of collision theory
- searching the Internet for relevant mathematical models
Answer to question 9
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0002) Computer simulations of a variety of chemistry topics are widely available. The visual and often interactive nature of these simulations makes them a valuable supplement to the teaching of conceptually challenging topics. In the case of the lab exercise, viewing a computer simulation of collision theory will strengthen the class's understanding of why temperature affects reaction rate. This review will aid them in analyzing and interpreting their results.
Correct Response: C. (Objective 0002) Computer simulations of a variety of chemistry topics are widely available. The visual and often interactive nature of these simulations makes them a valuable supplement to the teaching of conceptually challenging topics. In the case of the lab exercise, viewing a computer simulation of collision theory will strengthen the class's understanding of why temperature affects reaction rate. This review will aid them in analyzing and interpreting their results.
Question 10
10. A chemist wants to synthesize a certain chemical. The chemist begins with 2.15
g
of starting material and expects to have a 67% yield. Which of the following values most appropriately represents the amount of synthesized material that the chemist can expect to obtain?
- 1.4
g
- 1.44
g
- 1.440
g
- 1.4405
g
Answer to question 10
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0002) To determine the amount of synthesized material that is obtained, the starting mass of material is multiplied by the percent yield divided by 100: amount obtained = 2.15 * (67/100). When this is solved, the mathematical answer is 1.4405 g. However, the amount that can be expected to be obtained is dependent on the number of significant figures that the chemist used to calculate that value. The number of significant figures that a given value has is the number of known digits. These are the total number of digits before and after the decimal. For example, 2.15 g has three significant figures while 67% has two significant figures. The final amount obtained can only be expected up to the least number of significant figures that a starting value had. This is because the chemist can only be sure of the amount of material obtained in as many figures as it was known originally. Therefore, the expected amount can only be up to two significant figures, or 1.4 g.
Correct Response: A. (Objective 0002) To determine the amount of synthesized material that is obtained, the starting mass of material is multiplied by the percent yield divided by 100, or the amount obtained equals 2.15 multiplied by open parenthesis 67 divided by 100 close parenthesis. When this is solved, the mathematical answer is 1.4405 g. However, the amount that can be expected to be obtained is dependent upon the number of significant figures that the chemist used to calculate that value. The number of significant figures that a given value has is the number of known digits. These are the total number of digits before and after the decimal. For example, 2.15 g has three significant figures while 67% has two significant figures. The final amount obtained can only be expected up to the least number of significant figures that a starting value had. This is because the chemist can only be sure of the amount of material obtained in as many figures as it was known originally. Therefore, the expected amount can only be up to two significant figures, or 1.4 g.
Question 11
11. A chemistry teacher plans the student activities listed below as part of a new unit of study.
- comparing new terminology with related terminology from previous units
- developing nonverbal representations (e.g., charts, illustrations) of new terminology
- classifying new terminology according to specific criteria
- generating analogies with new terminology
These activities are likely to promote students' reading comprehension related to this unit primarily in which of the following ways?
- by providing the students with strategies for determining the meaning of unfamiliar vocabulary as they read
- by promoting the students' ability to decode and spell new vocabulary words accurately
- by teaching the students how to use structural analysis as a strategy for building domain-specific vocabulary
- by broadening the students' understanding of new vocabulary words and their associated concepts
Answer to question 11
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0003) Vocabulary knowledge is both a key indicator and predictor of reading comprehension ability. Vocabulary knowledge and concept development are closely interrelated; discipline-specific vocabulary development involves concept learning, and concept learning supports academic vocabulary development. Vocabulary development is an incremental process; more exposure to and opportunities to use new vocabulary in context result in greater depth of understanding. The instructional activities used by the teacher provide students with multiple opportunities to use the vocabulary from a new unit of study in context, thus promoting the students' vocabulary development and strengthening their understanding of related concepts.
Correct Response: D. (Objective 0003) Vocabulary knowledge is both a key indicator and predictor of reading comprehension ability. Vocabulary knowledge and concept development are closely interrelated; discipline-specific vocabulary development involves concept learning, and concept learning supports academic vocabulary development. Vocabulary development is an incremental process; more exposure to and opportunities to use new vocabulary in context result in greater depth of understanding. The instructional activities used by the teacher provide students with multiple opportunities to use the vocabulary from a new unit of study in context, thus promoting the students' vocabulary development and strengthening their understanding of related concepts.
Question 12
12. Some students in a chemistry class are English language learners and/or struggling readers who lack the basic reading skills necessary to fully comprehend assigned readings. Which of the following strategies would be most appropriate for the chemistry teacher to use to scaffold the reading comprehension of these students?
- providing the students with oral previews of text content and noting key concepts on the board
- having the students conduct alternative content research using online texts rather than printed ones
- teaching the students the basic reading skills they lack and reviewing key skills before assignments
- substituting lower-level chemistry texts for the students' reading assignments
Answer to question 12
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0003) Teachers need to provide scaffolding to English language learners and struggling readers who lack the basic reading skills necessary to comprehend assigned content-area readings. An oral preview of the content covered in a class reading assignment provides scaffolding by activating students' prior knowledge related to a text and building needed vocabulary and background knowledge required to make inferences and repair comprehension during reading. Noting key concepts on the board during the oral preview both reinforces the new concepts and vocabulary and helps students become familiar with how these elements will appear in print.
Correct Response: A. (Objective 0003) Teachers need to provide scaffolding to English language learners and struggling readers who lack the basic reading skills necessary to comprehend assigned content-area readings. An oral preview of the content covered in a class reading assignment provides scaffolding by activating students' prior knowledge related to a text and building needed vocabulary and background knowledge required to make inferences and repair comprehension during reading. Noting key concepts on the board during the oral preview both reinforces the new concepts and vocabulary and helps students become familiar with how these elements will appear in print.
Question 13
13. During a unit on nuclear energy, a chemistry teacher distributes two articles to students as a strategy for strengthening critical literacy skills. In one article, the author takes a stand strongly supporting the use of nuclear energy as an alternative to fossil fuels for providing heat and electricity. The author of the other article strongly opposes using nuclear energy for these purposes. Which of the following questions should the teacher ask students to first consider as they begin to analyze these readings?
- Which author makes the most effective argument for his or her position?
- Does the author have a financial connection to any company in the energy industry?
- Has the author properly cited sources for scientific content in the paper?
- Does the author anticipate and address arguments made by the opposition?
Answer to question 13
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0003) Identifying bias is a key component of critical reading. Before reading a persuasive argument, it is helpful for the reader to know whether the author has a predisposition to favor a particular position. Such information would include whether the author works for or is being paid by a particular company for their writing, as this could influence the objectivity of the author.
Correct Response: B. (Objective 0003) Identifying bias is a key component of critical reading. Before reading a persuasive argument, it is helpful for the reader to know whether the author has a predisposition to favor a particular position. Such information would include whether the author works for or is being paid by a particular company for their writing, as this could influence the objectivity of the author.
Question 14
14. Which of the following pedagogical strategies would best support students in improving their comprehension of content in their chemistry textbook?
- The teacher previews a chapter with students and reveals how to identify key points.
- The teacher develops focus questions for students to answer while reading an assigned chapter.
- The teacher assigns students to read a chapter in groups of two or three during class.
- The teacher directs students to read several pages of a chapter and then summarizes the key points.
Answer to question 14
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0003) Students should be provided with several strategies to help them comprehend a scientific text before they are assigned a reading. A preview can be done with the whole class looking at the text together with the teacher or by looking at templates for key text information. By previewing a reading in advance, the teacher can help students find many features of the text, such as the identification of key concepts, which will help them understand the content and use the textbook to learn independently. Such discussions could also include information such as how the graphics complement the text and how reading the captions provides helpful information about content.
Correct Response: A. (Objective 0003) Students should be provided with several strategies to help them comprehend a scientific text before they are assigned a reading. A preview can be done with the whole class looking at the text together with the teacher or by looking at templates for key text information. By previewing a reading in advance, the teacher can help students find many features of the text, such as the identification of key concepts, which will help them understand the content and use the textbook to learn independently. Such discussions could also include information such as how the graphics complement the text and how reading the captions provides helpful information about content.
Question 15
15. Which of the following compounds is paired with its correct Lewis dot structure?
-
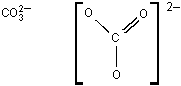 The Lewis dot structure is as follows; One central capital C atom is bonded to 3 individual capital O atoms. Two of the C O bonds are single bonds and one C O bond is a double bond. The whole structure is framed by a large set of brackets. On the outside of the brackets, on the upper right side, there is a negative 2.
The Lewis dot structure is as follows; One central capital C atom is bonded to 3 individual capital O atoms. Two of the C O bonds are single bonds and one C O bond is a double bond. The whole structure is framed by a large set of brackets. On the outside of the brackets, on the upper right side, there is a negative 2.
-
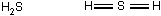 The Lewis dot structure is as follows; The three atoms in this compound are arranged in a linear fashion in the order capital H, capital S, capital H. Each S H bond is a double bond.
The Lewis dot structure is as follows; The three atoms in this compound are arranged in a linear fashion in the order capital H, capital S, capital H. Each S H bond is a double bond.
-
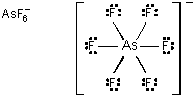 The Lewis dot structure is as follows; One central capital A lowercase S atom is bonded to six individual capital F atoms. Each AsF bond is a single bond. Each F atom is surrounded by six dots. The whole structure is framed by a large set of brackets. On the outside of the brackets, on the upper right side, there is a negative one.
The Lewis dot structure is as follows; One central capital A lowercase S atom is bonded to six individual capital F atoms. Each AsF bond is a single bond. Each F atom is surrounded by six dots. The whole structure is framed by a large set of brackets. On the outside of the brackets, on the upper right side, there is a negative one.
-
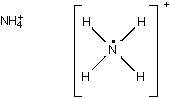 The Lewis dot structure is as follows; One central capital N atom is bonded to four individual capital H atoms. Each N H bond is a single bond. In addition to the four bond lines, the capital N atom also has one dot. The whole structure is framed by a large set of brackets. On the outside of the brackets, on the upper right side, there is a positive one.
The Lewis dot structure is as follows; One central capital N atom is bonded to four individual capital H atoms. Each N H bond is a single bond. In addition to the four bond lines, the capital N atom also has one dot. The whole structure is framed by a large set of brackets. On the outside of the brackets, on the upper right side, there is a positive one.
Answer to question 15
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0004) In order to determine the Lewis dot structure, it is first necessary to determine the number of valence electrons in the compound of interest. The arsenic hexafluoride ion shown has 48 valence electrons: 5 valence electrons associated with arsenic (As) due to its being a Group 5 element, 7 valence electrons for each of the Group 7 fluoride atoms (for a total of 42), and an extra electron because of the –1 charge on the ion. Each of the 6 fluoride atoms will be bonded to the central arsenic atom with an arrangement of 2 valence electrons per bond. This bond is represented by a single line between the atoms. The remaining 36 valence electrons are used to make a stable octet around each of the fluoride atoms, with 6 electrons associated with each atom. Each single electron in these octets is represented by a dot. The charge outside the brackets represents the negative ionic state of the arsenic hexafluoride ion.
Correct Response: C. (Objective 0004) In order to determine the Lewis dot structure, it is first necessary to determine the number of valence electrons in the compound of interest. The arsenic hexafluoride ion shown has 48 valence electrons: 5 valence electrons associated with arsenic (As) due to its being a Group 5 element, 7 valence electrons for each of the Group 7 fluoride atoms (for a total of 42), and an extra electron because of the negative 1 charge on the ion. Each of the 6 fluoride atoms will be bonded to the central arsenic atom with an arrangement of 2 valence electrons per bond. This bond is represented by a single line between the atoms. The remaining 36 valence electrons are used to make a stable octet around each of the fluoride atoms, with 6 electrons associated with each atom. Each single electron in these octets is represented by a dot. The charge outside the brackets represents the negative ionic state of the arsenic hexafluoride ion
Question 16
16. An electron in a multielectron atom has the quantum numbers shown below.
| Quantum Numbers |
| n |
ℓ |
mℓ |
ms |
| 3 |
1 |
0 |
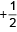 |
According to these numbers, what is known about this electron?
- It has acquired a positive charge.
- It is most likely found right next to the nucleus.
- It occupies a p-shaped orbital.
- It moves among three orbitals in the same subshell.
Answer to question 16
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0004) Quantum numbers describe the most probable location of an electron in an atom. The principle quantum number (n) provides information on the electron's distance from the nucleus and its energy level. The angular momentum quantum number or azimuthal quantum number (ℓ) describes the type of orbital. The magnetic quantum number (mℓ) describes the orbital's orientation in space and the electron spin quantum number (ms) describes the direction of the electron's spin. The quantum numbers for the given electron indicate that this electron occupies a 3p orbital.
Correct Response: C. (Objective 0004) Quantum numbers describe the most probable location of an electron in an atom. The principle quantum number open parenthesis n close parenthesis provides information on the electron's distance from the nucleus and its energy level. The angular momentum quantum number or azimuthal quantum number open parenthesis ℓ close parenthesis describes the type of orbital. The magnetic quantum number open parenthesis m sub ℓ close parenthesis describes the orbital's orientation in space and the electron spin quantum number open parenthesis m sub s close parenthesis describes the direction of the electron's spin. The quantum numbers for the given electron indicate that this electron occupies a 3 p orbital.
Question 17
17. The electron configurations of four atoms are shown in the table below.
| Atom |
Electron
Configuration |
| 1 |
1s2 |
| 2 |
1s22s1 |
| 3 |
1s22s2 |
| 4 |
1s22s22p6 |
According to these electron configurations, which atom is most likely to be attracted by a magnetic field?
- 1
- 2
- 3
- 4
Answer to question 17
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0004) Substances with one or more unpaired electrons will be attracted by a magnetic field. This property is known as paramagnetism. The electron configurations shown indicate that atom 2 is the only atom with an unpaired electron (in the 2s orbital). This suggests that atom 2 is the atom most likely to be attracted by a magnetic field.
Correct Response: B. (Objective 0004) Substances with one or more unpaired electrons will be attracted by a magnetic field. This property is known as paramagnetism. The electron configurations shown indicate that atom 2 is the only atom with an unpaired electron (in the 2s orbital). This suggests that atom 2 is the atom most likely to be attracted by a magnetic field.
Question 18
18. The structure of nitric acid (HNO3) is best described as a resonance hybrid of two possible Lewis structures. Which of the following best represents one of these Lewis structures?
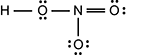 The oxygen is singly bonded to an nitrogen, which is doubly bonded to another oxygen with two lone pairs. In addition, the nitrogen is also singly binded to a third oxygen which has three lone pairs.
The oxygen is singly bonded to an nitrogen, which is doubly bonded to another oxygen with two lone pairs. In addition, the nitrogen is also singly binded to a third oxygen which has three lone pairs.
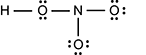 The oxygen is singly bonded to an nitrogen, which is singly bonded to another oxygen with three lone pairs. In addition, the nitrogen is also singly binded to a third oxygen which has three lone pairs.
The oxygen is singly bonded to an nitrogen, which is singly bonded to another oxygen with three lone pairs. In addition, the nitrogen is also singly binded to a third oxygen which has three lone pairs.
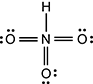 It is doubly bound on three of the sides to three oxygen atoms, and each oxygen atom has two lone pairs. In addition, the nitrogen is singly bound to a hydrogen atom.
It is doubly bound on three of the sides to three oxygen atoms, and each oxygen atom has two lone pairs. In addition, the nitrogen is singly bound to a hydrogen atom.
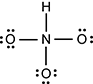 It is singly bound on three of the sides to three oxygen atoms, and each oxygen atom has three lone pairs. In addition, the nitrogen is singly bound to a hydrogen atom.
It is singly bound on three of the sides to three oxygen atoms, and each oxygen atom has three lone pairs. In addition, the nitrogen is singly bound to a hydrogen atom.
Answer to question 18
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0004) Nitric acid is a compound whose actual bond lengths and bond angles are not accurately described by a single Lewis structure. Instead, its structure more closely resembles a hybrid of two possible Lewis structures. One of those structures, represented by the correct response, is one in which the central N atom is surrounded by three O atoms (two with single bonds and one with a double bond). The other possible Lewis structure for nitric acid is one in which the double bond occurs between N and the other O atom not bonded to H.
Correct Response: A. (Objective 0004) Nitric acid is a compound whose actual bond lengths and bond angles are not accurately described by a single Lewis structure. Instead, its structure more closely resembles a hybrid of two possible Lewis structures. One of those structures, represented by the correct response, is one in which the central N atom is surrounded by three O atoms (two with single bonds and one with a double bond). The other possible Lewis structure for nitric acid is one in which the double bond occurs between upper N and the other upper O atom not bonded to upper H.
Question 19
19. Which of the following is the orbital diagram for sulfur?
-
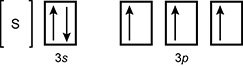 reading left to right open bracket s close bracket, box labeled three s with one up arrow and one down arrow, set of three boxes each with one up arrow in them and they are labeled three p
reading left to right open bracket s close bracket, box labeled three s with one up arrow and one down arrow, set of three boxes each with one up arrow in them and they are labeled three p
-
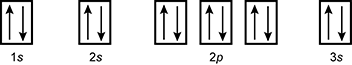 reading left to right box labeled one s with one up arrow and one down arrow, box labeled two s with one up arrow and one down arrow, set of three boxes each with one up arrow and one down arrow labeled two p and then one box labeled three s with one up arrow and one down arrow
reading left to right box labeled one s with one up arrow and one down arrow, box labeled two s with one up arrow and one down arrow, set of three boxes each with one up arrow and one down arrow labeled two p and then one box labeled three s with one up arrow and one down arrow
-
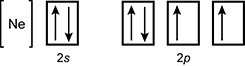 reading left to right open bracket upper n e close bracket box labeled two s with one up arrow and one down arrow set of three boxes labeled two p where the first box has an up arrow and a down arrow and the other two boxes have one up arrow in them
reading left to right open bracket upper n e close bracket box labeled two s with one up arrow and one down arrow set of three boxes labeled two p where the first box has an up arrow and a down arrow and the other two boxes have one up arrow in them
-
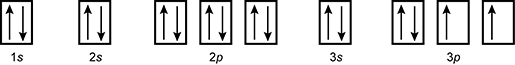 reading left to right box labeled one s with one up arrow and one down arrow box labeled two s with one up arrow and one down arrow set of three boxes labeled two p where each box has one up arrow and one down arrow box labeled three s with one up arrow and one down arrow set of three boxes labeled three p where the first box has an up arrow and a down arrow and the other two boxes have one up arrow in them
reading left to right box labeled one s with one up arrow and one down arrow box labeled two s with one up arrow and one down arrow set of three boxes labeled two p where each box has one up arrow and one down arrow box labeled three s with one up arrow and one down arrow set of three boxes labeled three p where the first box has an up arrow and a down arrow and the other two boxes have one up arrow in them
Answer to question 19
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0004) Orbital diagrams are representations of an atom's electron configuration. The boxes represent the different orbitals and the single arrows represent individual electrons. For atoms with electrons in p, d, or f subshells, Hund's rule is used to determine the order of filling. The electron configuration of sulfur is 1s22s22p63s23p4. This electron configuration is best represented by the orbital diagram that includes four electrons in the 3p orbital.
Correct Response: D. (Objective 0004) Orbital diagrams are representations of an atom's electron configuration. The boxes represent the different orbitals and the single arrows represent individual electrons. For atoms with electrons in p, d, or f subshells, Hund's rule is used to determine the order of filling. The electron configuration of sulfur is 1 s squared 2 s squared 2 p sup six baseline 3 s squared 3 p sup four. This electron configuration is best represented by the orbital diagram that includes four electrons in the 3 p orbital.
Question 20
20. An ionic compound is most likely to form when a Group 1 element is reacted with an element from Group:
- 2.
- 6.
- 11.
- 17.
Answer to question 20
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0005) Ionic compounds are formed when electrons are transferred from one element to another, resulting in the formation of an ionic bond. This type of bond occurs between metallic and nonmetallic elements. Elements in Group 1, which includes such elements as lithium and sodium, are alkali metals and tend to lose one electron, thereby becoming positively charged. Consequently, these elements will form an ionic bond with nonmetals that tend to gain a single electron and become negatively charged, including elements found in Group 17, which includes fluorine and chlorine.
Correct Response: D. (Objective 0005) Ionic compounds are formed when electrons are transferred from one element to another, resulting in the formation of an ionic bond. This type of bond occurs between metallic and nonmetallic elements. Elements in Group 1, which includes such elements as lithium and sodium, are alkali metals and tend to lose one electron, thereby becoming positively charged. Consequently, these elements will form an ionic bond with nonmetals that tend to gain a single electron and become negatively charged, including elements found in Group 17, which includes fluorine and chlorine.
Question 21
21. Which of the following Period 2 elements has the greatest atomic radius?
- Li
- B
- C
- F
Answer to question 21
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0005) While the number of protons increases from left to right for elements within the same period, the number of inner shell electrons stays the same. As the number of protons increases across a period, the effective nuclear charge increases. As a result, the valence electrons are attracted more strongly to the positive charge of the nucleus and the atomic radius is less. This explains why the atomic radius of main group elements in the same period increases from right to left across the periodic table. Of the Period 2 elements listed, lithium will have the greatest atomic radius.
Correct Response: A. (Objective 0005) While the number of protons increases from left to right for elements within the same period, the number of inner shell electrons stays the same. As the number of protons increases across a period, the effective nuclear charge increases. As a result, the valence electrons are attracted more strongly to the positive charge of the nucleus and the atomic radius is less. This explains why the atomic radius of main group elements in the same period increases from right to left across the periodic table. Of the Period 2 elements listed, lithium will have the greatest atomic radius.
Question 22
22. H, Li, Na, K
The Group 1 atoms shown above follow the general periodic trend in electron affinity. Which of these Group 1 atoms has the weakest affinity for electrons?
- H
- Li
- Na
- K
Answer to question 22
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0005) Electron affinity is a measure of how easily an atom gains an electron to form an anion. In general, electron affinity increases from the bottom to the top of the periodic table and from left to right across the periodic table. These trends are related to the electron configurations of the elements. All of the Group 1 atoms listed have one valence electron in an s orbital (H = 1s, Li = 3s, Na = 2s, and K = 4s). As the principal quantum number increases, the relative distance between an added electron and the nucleus increases. The increased distance plus the presence of more core electrons results in a reduced attraction between the positive nucleus and an added electron. This reduced attraction between the nucleus and an added electron corresponds to a weaker electron affinity. Of the Group 1 atoms listed, K, which has the greatest principal quantum number and the most core electrons, has the weakest affinity for electrons.
Correct Response: D. (Objective 0005) Electron affinity is a measure of how easily an atom gains an electron to form an anion. In general, electron affinity increases from the bottom to the top of the periodic table and from left to right across the periodic table. These trends are related to the electron configurations of the elements. All of the Group 1 atoms listed have one valence electron in an s orbital open parenthesis upper h equals 1 s, upper l i equals 3 s, upper n a equals 2 s, and upper K equals 4 s close parenthesis. As the principal quantum number increases, the relative distance between an added electron and the nucleus increases. The increased distance plus the presence of more core electrons results in a reduced attraction between the positive nucleus and an added electron. This reduced attraction between the nucleus and an added electron corresponds to a weaker electron affinity. Of the Group 1 atoms listed, upper K, which has the greatest principal quantum number and the most core electrons, has the weakest affinity for electrons.
Question 23
23. In Period 2 of the periodic table, which of the following atoms is of the smallest size?
- Lithium
- Boron
- Nitrogen
- Fluorine
Answer to question 23
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0005) Of the four elements listed, Fluorine has the highest atomic number. Since it has the highest atomic number, it also has the greatest number of protons. However, it is not a large atom because of nuclear force. Nuclear force holds together the protons and neutrons of a nucleus. The stronger the nuclear force, the more closely that the electrons will be attracted to the nucleus.
Correct Response: D. (Objective 0005) Of the four elements listed, Fluorine has the highest atomic number. Since it has the highest atomic number, it also has the greatest number of protons. However, it is not a large atom because of nuclear force. Nuclear force holds together the protons and neutrons of a nucleus. The stronger the nuclear force, the more closely that the electrons will be attracted to the nucleus.
Question 24
24. Use the graph below to answer the question that follows.
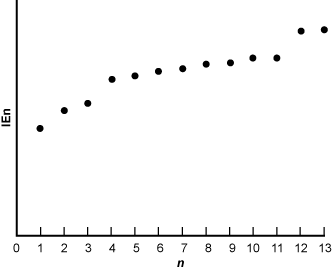
The electron 1 has an ionization energy about halfway up the y-axis. The electrons 2 and 3 have slightly higher ionization energies. There is a jump up to electron 4 then electrons 4 through 11 have ionization energies that are only slightly above each other so that 11 is the highest ionization energy. There is another large jump to electron 12, which is roughly tied with electron 13 for highest ionization energy.
The graph shows a plot of natural logarithms of ionization energy, IEn, against the number of electrons removed, n, for a certain element. The element that is represented in the graph can be identified because the graph suggests the presence of:
- a 3p subshell with three electrons.
- a full 2s subshell with the most energy needed to remove the last two electrons.
- three subshells and three valence electrons.
- three subshells that are filled such that it is more difficult to add new electrons.
Answer to question 24
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0005) This graph shows a trend in electron removal for a certain element: as more electrons are removed, the amount of energy it takes to remove the next electron increases. This amount of energy is called ionization energy. The greater the ionization energy, the more stable a given electron. In general, ionization energy increases from left to right among rows and from bottom to top of columns in the periodic table. In this graph, there are two larger spikes in ionization energy that occur before the two far right electrons and after the three leftmost electrons. Both of these represent the end of a filled subshell, the 1s and 2s subshells, respectively. This means that there are three electrons in addition to the 2s subshell, making the element aluminum.
Correct Response: C. (Objective 0005) This graph shows a trend in electron removal for a certain element: as more electrons are removed, the amount of energy it takes to remove the next electron increases. This amount of energy is called ionization energy. The greater the ionization energy, the more stable a given electron. In general, ionization energy increases from left to right among rows and from bottom to top of columns in the periodic table. In this graph, there are two larger spikes in ionization energy that occur before the two far right electrons and after the three leftmost electrons. Both of these represent the end of a filled subshell, the 1s and 2s subshells, respectively. This means that there are three electrons in addition to the 2s subshell, making the element aluminum.
Question 25
25. Use the passage below to answer the question that follows.
Mineral compounds are ionic compounds with variable compositions in which one or two ions may be substituted in the crystal lattice of the mineral. For example, under the right conditions of temperature and pressure, Na and Al ions replace Ca and Mg ions in diopside (CaMgSi2O6) to form jadeite (NaAlSi2O6).Mineral compounds are ionic compounds with variable compositions in which one or two ions may be substituted in the crystal lattice of the mineral. For example, under the right conditions of temperature and pressure, capital N lowercase A and capital A lowercase L ions replace capital C lowercase A and capital M lowercase G ions in diopside, capital C lowercase A capital M lowercase G capital S lowercase I subscript 2 capital O subscript 6, to form jadeite, capital N lowercase A capital A lowercase L capital S lowercase I subscript 2 capital O subscript 6.
The ions of which of the following atoms most likely completely substitute for Fe in siderite, Fe(CO)3, under the right environmental conditions?The ions of which of the following atoms most likely completely substitutes for capital F lowercase E in siderite, capital F lowercase E open parenthesis capital C O close parenthesis subscript 3, under the right environmental conditions?
- Licapital L lowercase I
- Mncapital M lowercase N
- Secapital S lowercase E
- Ptcapital P lowercase T
Answer to question 25
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0005) The sum of the charges in an ionic compound is zero. Therefore, the sum of charges in diopside and jadeite are equal. Thus, when atoms are substituted, the most likely atoms to be substituted are those that will allow the crystalline compound to maintain its net charge. In the case of diopside, Ca2+ and Mg2+ are replaced with Na+ and Al3+, or 2 + 2 = 1 + 3. Additionally, the atoms that are substituted must have a similar atomic radii to the original atoms, frequently making them close on the periodic table. The Fe in siderite has a +2 charge. Therefore, Mn is the most likely atom to be substituted.
Correct Response: B. (Objective 0005) The sum of the charges in an ionic compound is zero. Therefore, the sum of charges in diopside and jadeite are equal. Thus, when atoms are substituted, the most likely atoms to be substituted are those that will allow the crystalline compound to maintain its net charge. In the case of diopside, capital C lowercase A superscript 2 plus and capital M lowercase G superscript 2 plus are replaced with capital N lowercase A superscript plus and capital A lowercase L superscript three plus or 2 + 2 equals 1 + 3. Additionally, the atoms that are substituted must have a similar atomic radii to the original atoms, frequently making them close on the periodic table. The capital F lowercase E in siderite has a positive two charge. Therefore, capital M lowercase N is the most likely atom to be substituted.
Question 26
26.
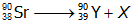
In the reaction above showing the nuclear decay of strontium-90 to yttrium-90, X represents:
- a positron.
- an alpha particle.
- a neutron.
- a beta particle.
Answer to question 26
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0006) The type of particle represented by X can be determined by first balancing the given nuclear equation. In a balanced nuclear equation, the sum of the mass numbers and the sum of the atomic numbers are the same on both sides of the equation. The mass number in this reaction is the same on both sides of the equation, but the atomic number changes from 38 to 39. Therefore, the mass number of X must be 0 and the atomic number of X must be –1, giving X the characteristics of a beta particle. Beta particles are high-speed electrons and are represented in nuclear equations by
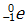 or
or
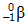 .
Correct Response: D. (Objective 0006) The type of particle represented by X can be determined by first balancing the given nuclear equation. In a balanced nuclear equation, the sum of the mass numbers and the sum of the atomic numbers are the same on both sides of the equation. The mass number in this reaction is the same on both sides of the equation, but the atomic number changes from 38 to 39. Therefore, the mass number of X must be 0 and the atomic number of X must be negative 1, giving X the characteristics of a beta particle. Beta particles are high-speed electrons and are represented in nuclear equations by sup zero sub negative one baseline e or sup zero sub negative one baseline beta.
.
Correct Response: D. (Objective 0006) The type of particle represented by X can be determined by first balancing the given nuclear equation. In a balanced nuclear equation, the sum of the mass numbers and the sum of the atomic numbers are the same on both sides of the equation. The mass number in this reaction is the same on both sides of the equation, but the atomic number changes from 38 to 39. Therefore, the mass number of X must be 0 and the atomic number of X must be negative 1, giving X the characteristics of a beta particle. Beta particles are high-speed electrons and are represented in nuclear equations by sup zero sub negative one baseline e or sup zero sub negative one baseline beta.
Question 27
27. The graph below shows the number of protons and the number of neutrons present in various stable isotopes.
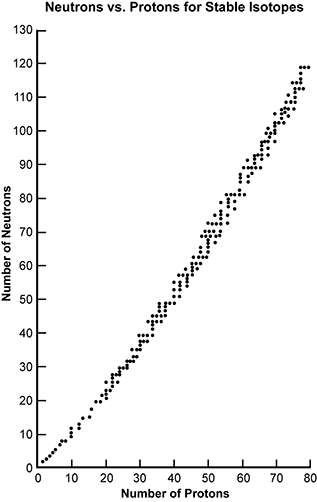
the graph has a large number of data points starting close to the origin in the first quadrant and increases pretty steadily where when the number of protons increase so does the number of neutrons.
If an isotope with an atomic number greater than 30 has a neutron-to-proton ratio that falls below this belt of stable isotopes, which of the following modes of radioactive decay is this isotope most likely to undergo?
- positron emission
- alpha emission
- gamma ray emission
- beta emission
Answer to question 27
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0006) The graph of neutrons and protons in various stable atomic nuclei can be used to help predict the stability of an isotope and to predict the most probable mode of radioactive decay. In this case, the neutron-to-proton ratio for the isotope falls below the belt of stability. This means its neutron-to-proton ratio is lower than those of stable isotopes with the same number of protons. For the isotope to raise its neutron-to-proton ratio, the number of protons must decrease while the atomic mass remains unchanged. There are two modes of radioactive decay that can achieve this result—positron emission and electron capture.
Correct Response: A. (Objective 0006) The graph of neutrons and protons in various stable atomic nuclei can be used to help predict the stability of an isotope and to predict the most probable mode of radioactive decay. In this case, the neutron-to-proton ratio for the isotope falls below the belt of stability. This means its neutron-to-proton ratio is lower than those of stable isotopes with the same number of protons. For the isotope to raise its neutron-to-proton ratio, the number of protons must decrease while the atomic mass remains unchanged. There are two modes of radioactive decay that can achieve this result—positron emission and electron capture.
Question 28
28. The radioisotope tritium has a half-life of 12.3 years. How long will it take for a 100 μg sample of tritium to decay to 12.5% of its original activity?
- 12.3 years
- 24.6 years
- 36.9 years
- 49.2 years
Answer to question 28
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0006) The activity of the tritium sample is directly related to the mass of sample remaining. This 100 μg sample will have 12.5% of its activity remaining when there are 12.5 μg of tritium left. Since each half-life reduces the mass of the sample by one-half, the tritium sample will have 12.5% of its original activity after three half-lives (i.e., 100 μg to 50 μg to 25 μg to 12.5 μg). Three half-lives for tritium equal 36.9 years (i.e., 3 × 12.3 years).
Correct Response: C. (Objective 0006) The activity of the tritium sample is directly related to the mass of sample remaining. This 100 microgram sample will have twelve point five percent of its activity remaining when there are 12.5 microgram of tritium left. Since each half-life reduces the mass of the sample by one-half, the tritium sample will have twelve point five percent of its original activity after three half-lives (i.e., 100 μ g to 50 μ g to 25 μ g to 12.5 μ g). Three half-lives for tritium equal 36.9 years (i.e., 3 times 12.3 years).
Question 29
Use the equation below to answer the question that follows.
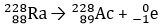 On the left of the reaction arrow is the chemical species capital R lowercase A with the numbers 228 above 88 to the left of the species. On the right of the reaction arrow is the chemical species capital A lowercase C with the numbers 228 above 89 to the left of the species. There is also another chemical species that is a lowercase E with the numbers 0 above negative 1 to the left of the species.
On the left of the reaction arrow is the chemical species capital R lowercase A with the numbers 228 above 88 to the left of the species. On the right of the reaction arrow is the chemical species capital A lowercase C with the numbers 228 above 89 to the left of the species. There is also another chemical species that is a lowercase E with the numbers 0 above negative 1 to the left of the species.
29.
The nuclear reaction represented above is best classified as:
- nuclear fusion.
- beta decay.
- nuclear fission.
- alpha decay.
Answer to question 29
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0006) A beta particle is a fast-moving electron that can be emitted from the nucleus of a radioactive isotope during the decay process. In this case, a neutron in radium (Ra) has decayed, releasing a proton and an electron. The proton raises the atomic number to 89, which is that of actinium (Ac). The electron is released, as indicated by
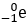 , shown as a product of this reaction.
Correct Response: B. (Objective 0006) A beta particle is a fast-moving electron that can be emitted from the nucleus of a radioactive isotope during the decay process. In this case, a neutron in radium, capital R lowercase A, has decayed, releasing a proton and an electron. The proton raises the atomic number to 89, which is that of actinium, capital A lowercase C. The electron is released, as indicated by lowercase E with the numbers 0 above negative 1 to its left, shown as a product of this reaction.
, shown as a product of this reaction.
Correct Response: B. (Objective 0006) A beta particle is a fast-moving electron that can be emitted from the nucleus of a radioactive isotope during the decay process. In this case, a neutron in radium, capital R lowercase A, has decayed, releasing a proton and an electron. The proton raises the atomic number to 89, which is that of actinium, capital A lowercase C. The electron is released, as indicated by lowercase E with the numbers 0 above negative 1 to its left, shown as a product of this reaction.
Question 30
30. A scientist adds 2.50 mL of toluene (C7H8) to a reaction mixture at 25°C. Given that the density of toluene is 0.8623 g/mL at 25°C, how many molecules of toluene were added to the mixture?
- 1.26 × 1022
- 1.41 × 1022
- 1.63 × 1022
- 1.89 × 1022
Answer to question 30
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0007) The number of molecules of toluene added to the reaction mixture can be determined using the given volume of toluene, its density, its molar mass, and Avogadro's number. The mass of toluene added to the mixture is calculated by multiplying the volume times the density (2.50 mL × 0.8623 g/mL = 2.16 g). The molar mass of toluene is 92.15 g/mol and is calculated by adding the atomic masses of each atom type in 1 mol of toluene (7 C = 84.07 g, 8 H = 8.08 g). The number of moles of toluene can then be determined by dividing the mass of toluene by its molar mass (2.16 g ÷ 92.15 g/mol = 0.0234 mol of toluene). To convert from moles to molecules of toluene, 0.0234 mol is multiplied by Avogadro's number (6.02 × 1023 molecules/mol). These calculations determine that 1.41 × 1022 molecules of toluene were added to the reaction mixture.
Correct Response: B. (Objective 0007) The number of molecules of toluene added to the reaction mixture can be determined using the given volume of toluene, its density, its molar mass, and Avogadro's number. The mass of toluene added to the mixture is calculated by multiplying the volume times the density open parenthesis 2.50 m L times 0.8623 g slash m L equals 2.16 g). The molar mass of toluene is 92.15 g slash mol and is calculated by adding the atomic masses of each atom type in 1 mol of toluene open parenthesis 7 upper C equals 84.07 g, 8 upper H equals 8.08 g close parenthesis. The number of moles of toluene can then be determined by dividing the mass of toluene by its molar mass open parenthesis 2.16 g divided by 92.15 g slash mol equals 0.0234 mol of toluene close parenthesis. To convert from moles to molecules of toluene, 0.0234 mol is multiplied by Avogadro's number open parenthesis 6.02 times 10 sup twenty three molecules slash mol). These calculations determine that 1.41 times 10 sup twenty two molecules of toluene were added to the reaction mixture.
Question 31
31. A student wants to determine the molality of a 5.00% by mass aqueous solution of NaCl prepared using 10.0 g of NaCl and 190.0 mL of H2O at 20°C. Given this information, what is the molality of the prepared NaCl solution?
- 0.0162 m
- 0.0526 m
- 0.450 m
- 0.900 m
Answer to question 31
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0007) The concentration of a solution can be expressed as molality, molarity, percent by mass, or mole fraction. Molality (m) is equal to moles of solute divided by the mass of solvent in kg. In this example, there is 0.171 mol of NaCl
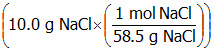 and 0.190 kg of H2O
and 0.190 kg of H2O
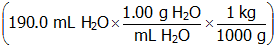 . Given these values, the molality of the NaCl solution is 0.900 m
. Given these values, the molality of the NaCl solution is 0.900 m
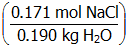 .
Correct Response: D. (Objective 0007) The concentration of a solution can be expressed as molality, molarity, percent by mass, or mole fraction. Molality (m) is equal to moles of solute divided by the mass of solvent in k g. In this example, there is 0.171 mol of Upper N a upper C l open parenthesis ten point zero g Upper n a upper c l time open parenthesis start fraction numerator one mol upper n a upper c l denominator fifty eight point five g upper n a upper c l end fraction close parenthesis close parenthesis and 0.190 k g of Upper H sub two upper O open parenthesis one hundred ninety point zero m l upper h two upper o times start fraction numerator one point zero zero g upper h two upper o denominator m l upper h two upper o end fraction times start fraction numerator one k g denominator one thousand g end fraction close parenthesis. Given these values, the molality of the upper N a upper C l solution is 0.900 m open parenthesis start fraction numerator zero point one seven one mol upper n a upper c l denominator zero point one nine zero k g upper h two upper o end fraction close parenthesis.
.
Correct Response: D. (Objective 0007) The concentration of a solution can be expressed as molality, molarity, percent by mass, or mole fraction. Molality (m) is equal to moles of solute divided by the mass of solvent in k g. In this example, there is 0.171 mol of Upper N a upper C l open parenthesis ten point zero g Upper n a upper c l time open parenthesis start fraction numerator one mol upper n a upper c l denominator fifty eight point five g upper n a upper c l end fraction close parenthesis close parenthesis and 0.190 k g of Upper H sub two upper O open parenthesis one hundred ninety point zero m l upper h two upper o times start fraction numerator one point zero zero g upper h two upper o denominator m l upper h two upper o end fraction times start fraction numerator one k g denominator one thousand g end fraction close parenthesis. Given these values, the molality of the upper N a upper C l solution is 0.900 m open parenthesis start fraction numerator zero point one seven one mol upper n a upper c l denominator zero point one nine zero k g upper h two upper o end fraction close parenthesis.
Question 32
32. How many grams of H are present in 52.5 g of carbonic acid (H2CO3)?
- 1.63 g
- 1.71 g
- 2.02 g
- 3.26 g
Answer to question 32
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0007) The first step in determining the mass of H present in 52.5 g of H2CO3 is determining the percent composition by mass of H. The chemical formula indicates that in 1 mol of H2CO3 there are 2 mol or 2.02 g of H. The following calculations can be used to determine the percent by mass of H in 1 mol of H2CO3:
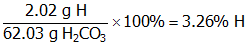 . This means that there are 3.26 g of H in every 100 g of H2CO3. This information can be used to determine the mass of H in 52.5 g of H2CO3
. This means that there are 3.26 g of H in every 100 g of H2CO3. This information can be used to determine the mass of H in 52.5 g of H2CO3
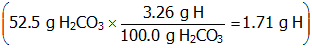 .
Correct Response: B. (Objective 0007) The first step in determining the mass of upper H present in 52.5 g of upper H sub two upper C upper O sub three is determining the percent composition by mass of upper H. The chemical formula indicates that in 1 mol of upper H sub two upper C upper O sub three there are 2 mol or 2.02 g of upper H. The following calculations can be used to determine the percent by mass of upper H in 1 mol of upper H sub two upper C upper O three: start fraction numerator two point zero two g upper h denominator sixty two point zero three g upper h two upper c upper o three end fraction times one hundred percent equals three point two six percent upper h. This means that there are 3.26 g of upper H in every 100 g of upper H sub two upper C upper O sub three. This information can be used to determine the mass of upper H in 52.5 g of upper H sub two upper C upper O sub three open parenthesis fifty two point five g upper h two upper c upper o three times start fraction numerator three point two six g upper h denominator one hundred point zero g upper h two upper c upper o three equals one point seven one g upper h.
.
Correct Response: B. (Objective 0007) The first step in determining the mass of upper H present in 52.5 g of upper H sub two upper C upper O sub three is determining the percent composition by mass of upper H. The chemical formula indicates that in 1 mol of upper H sub two upper C upper O sub three there are 2 mol or 2.02 g of upper H. The following calculations can be used to determine the percent by mass of upper H in 1 mol of upper H sub two upper C upper O three: start fraction numerator two point zero two g upper h denominator sixty two point zero three g upper h two upper c upper o three end fraction times one hundred percent equals three point two six percent upper h. This means that there are 3.26 g of upper H in every 100 g of upper H sub two upper C upper O sub three. This information can be used to determine the mass of upper H in 52.5 g of upper H sub two upper C upper O sub three open parenthesis fifty two point five g upper h two upper c upper o three times start fraction numerator three point two six g upper h denominator one hundred point zero g upper h two upper c upper o three equals one point seven one g upper h.
Question 33
33. A compound is 5.94% H by mass and 94.01% O by mass and has a molecular weight between 30.00 g and 35.00 g. What is the molecular formula of this compound?
- OH−
- H2O
- HO2
- H2O2
Answer to question 33
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0007) The molecular formula for a compound includes the numbers and types of atoms present in the compound. In this example, the percent composition by mass and information about the molecular weight of the compound are given. The percent composition of each atom can be used to determine the molar ratio of the two atoms. In a 100 g sample of the compound, there are 5.94 g of H and 94.01 g of O. When these mass values are converted to moles, the molar ratio of H:O is determined to be 5.88 mol H:5.88 mol O. This corresponds to a 1:1 ratio of H:O. However, HO is not the molecular formula for this compound because the molecular weight of HO is not between 30.00 g and 35.00 g. The molecular formula must exhibit a 1:1 ratio between the atom types and have a molecular weight within the given range. The molecular formula for hydrogen peroxide (H2O2) is the only listed compound that satisfies both of these criteria.
Correct Response: D. (Objective 0007) The molecular formula for a compound includes the numbers and types of atoms present in the compound. In this example, the percent composition by mass and information about the molecular weight of the compound are given. The percent composition of each atom can be used to determine the molar ratio of the two atoms. In a 100 g sample of the compound, there are 5.94 g of upper H and 94.01 g of upper O. When these mass values are converted to moles, the molar ratio of upper H : upper O is determined to be 5.88 mol upper H : 5.88 mol upper O. This corresponds to a 1:1 ratio of upper H : upper O. However, upper H upper O is not the molecular formula for this compound because the molecular weight of upper H upper O is not between 30.00 g and 35.00 g. The molecular formula must exhibit a 1:1 ratio between the atom types and have a molecular weight within the given range. The molecular formula for hydrogen peroxide open parenthesis upper H sub two upper O sub two close parenthesis is the only listed compound that satisfies both of these criteria.
Question 34
34. CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ) A chemical equation is shown and reads as follows; Capital C capital H subscript four, gas, reacts with two capital O subscript two, gas, to produce capital C capital O subscript two, gas, and two capital H subscript two capital O, liquid. ΔH = –890.3 kJ The enthalpy change for the reaction is equal to negative eight hundred ninety point three kilojoules.
Given the balanced equation for the combustion of methane shown above, how many moles of methane would need to be reacted in order to produce 3561 kJthree thousand five hundred sixty-one kilojoules of energy?
- 2
- 4
- 8
- 16
Answer to question 34
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0008) According to the balanced equation shown, the combustion of one mole of methane gives off 809.3 kJ of heat. In order to determine the number of moles of methane (CH4) that would need to react in order to produce 3561 kJ of energy, it is necessary to divide 3561 kJ by the amount produced by the combustion of a single mole of methane (890.3 kJ). In other words, x moles of methane • 890.3 kJ/1 mole CH4 = 3561 kJ of energy. Solving for the number of moles, x = 3561 kJ/890.3 kJ and x = 4.
Correct Response: B. (Objective 0008) According to the balanced equation shown, the combustion of one mole of methane gives off 809.3 kilo joules of heat. In order to determine the number of moles of methane ( capital C H subscript ) that would need to react in order to produce 3,561 kilo joules of energy, it is necessary to divide 3,561 kilo joules by the amount produced by the combustion of a single mole of methane (890.3 kilo joules ). In other words, X moles of methane times 890.3 kilo joules per 1 mole of methane equals 3 thousand 561 kilo joules of energy. Solving for the number of moles, x = 3 thousand 561 kilo joules divided by 890.3 kilo joules and X equals 4.
Question 35
35. C8H18(ℓ) + O2(g) → CO2(g) + H2O(g)
The chemical equation for the combustion of octane shown above is unbalanced. When this equation is balanced with the lowest set of whole number coefficients, which of the following is the stoichiometric coefficient for CO2?
- 8
- 12
- 16
- 20
Answer to question 35
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0008) In a balanced chemical equation there are the same numbers and types of atoms on the reactant side as there are on the product side. Using the lowest set of whole number coefficients, the balanced chemical equation for the combustion of octane is as follows: 2C8H18(ℓ) + 25O2(g) → 16CO2(g) + 18H2O(g). These coefficients correspond to 16 C atoms, 36 H atoms, and 50 O atoms on both sides of the reaction arrow.
Correct Response: C. (Objective 0008) In a balanced chemical equation there are the same numbers and types of atoms on the reactant side as there are on the product side. Using the lowest set of whole number coefficients, the balanced chemical equation for the combustion of octane is as follows: two upper C sub eight upper H sub eighteen open parenthesis ℓ close parenthesis plus twenty five upper o sub two open parenthesis g close parenthesis produces sixteen upper c upper o sub two open parenthesis g close parenthesis plus eighteen upper h sub two upper o open parenthesis g close parenthesis. These coefficients correspond to 16 upper C atoms, 36 upper H atoms, and 50 upper O atoms on both sides of the reaction arrow.
Question 36
36. 2Ca(s) + O2(g)
 2CaO(s)
2CaO(s)
According to the oxidation reaction shown above, what mass of CaO will be produced when 120.3 g of Ca react with excess O2?
- 240.6 g
- 168.3 g
- 120.3 g
- 112.2 g
Answer to question 36
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0008) The balanced chemical equation indicates a 1:1 stoichiometric ratio between Ca and CaO. Therefore, the oxidation of 120.3 g (or 3 mol) of Ca will produce 3 mol of CaO, which is equivalent to 168.3 g of CaO
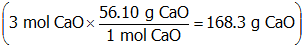 .
Correct Response: B. (Objective 0008) The balanced chemical equation indicates a 1:1 stoichiometric ratio between upper C a and upper C a upper O. Therefore, the oxidation of 120.3 g (or 3 mol) of upper C a will produce 3 mol of upper C a upper O, which is equivalent to 168.3 g of upper C a upper O open parenthesis three moles upper c a upper o times start fraction numerator fifty six point one zero g upper c a upper o denominator one mole upper c a upper o end fraction equals one hundred sixty eight point three g of upper c a upper o close parenthesis.
.
Correct Response: B. (Objective 0008) The balanced chemical equation indicates a 1:1 stoichiometric ratio between upper C a and upper C a upper O. Therefore, the oxidation of 120.3 g (or 3 mol) of upper C a will produce 3 mol of upper C a upper O, which is equivalent to 168.3 g of upper C a upper O open parenthesis three moles upper c a upper o times start fraction numerator fifty six point one zero g upper c a upper o denominator one mole upper c a upper o end fraction equals one hundred sixty eight point three g of upper c a upper o close parenthesis.
Question 37
37. Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq)
According to the chemical equation shown above, how many moles of PbI2 will be formed when 2.0 mol of Pb(NO3)2 are reacted with 3.0 mol of KI?
- 1.0 mol
- 1.5 mol
- 2.0 mol
- 2.5 mol
Answer to question 37
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0008) Identifying the limiting reactant is the first step in determining the number of moles of PbI2 formed. The stoichiometric ratio between the reactants and the amount of each reactant present can be used to determine which reactant is limiting. In this example, KI is the limiting reactant. This is because 2.0 mol of Pb(NO3)2 require 4.0 mol of KI to react completely and only 3.0 mol of KI are available. Once the limiting reactant has been identified, the amount of product can be calculated using the stoichiometric ratio between the limiting reactant and the desired product. In this example, 1.5 mol of PbI2 will be formed when 3.0 mol of KI are reacted with 2 mol of Pb(NO3)2
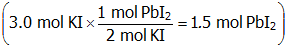 .
Correct Response: B. (Objective 0008) Identifying the limiting reactant is the first step in determining the number of moles of upper P b upper I sub two formed. The stoichiometric ratio between the reactants and the amount of each reactant present can be used to determine which reactant is limiting. In this example, upper K upper I is the limiting reactant. This is because 2.0 mol of upper P b open parenthesis upper n upper o sub three close parenthesis sub two require 4.0 mol of upper K upper I to react completely and only 3.0 mol of upper K upper I are available. Once the limiting reactant has been identified, the amount of product can be calculated using the stoichiometric ratio between the limiting reactant and the desired product. In this example, 1.5 mol of upper P b upper I sub two will be formed when 3.0 mol of upper K upper I are reacted with 2 mol of upper P b open parenthesis upper n upper o sub three close parenthesis sub two open parenthesis three point zero mole upper k upper i time start fraction numerator one mole upper p b upper i sub two denominator two mole upper k upper i end fraction equals one point five mole upper p b upper i sub two close parenthesis.
.
Correct Response: B. (Objective 0008) Identifying the limiting reactant is the first step in determining the number of moles of upper P b upper I sub two formed. The stoichiometric ratio between the reactants and the amount of each reactant present can be used to determine which reactant is limiting. In this example, upper K upper I is the limiting reactant. This is because 2.0 mol of upper P b open parenthesis upper n upper o sub three close parenthesis sub two require 4.0 mol of upper K upper I to react completely and only 3.0 mol of upper K upper I are available. Once the limiting reactant has been identified, the amount of product can be calculated using the stoichiometric ratio between the limiting reactant and the desired product. In this example, 1.5 mol of upper P b upper I sub two will be formed when 3.0 mol of upper K upper I are reacted with 2 mol of upper P b open parenthesis upper n upper o sub three close parenthesis sub two open parenthesis three point zero mole upper k upper i time start fraction numerator one mole upper p b upper i sub two denominator two mole upper k upper i end fraction equals one point five mole upper p b upper i sub two close parenthesis.
Question 38
38. 2Fe2O3(s) + 3C(s) → 4Fe(s) + 3CO2(g)
When 1 mol of Fe2O3 is reacted with excess C according to the chemical equation shown above, 91.5 g of Fe are produced. What is the percent yield of this reaction?
- 54.8%
- 41.0%
- 81.7%
- 91.9%
Answer to question 38
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0008) The percent yield for a reaction is defined as the
 . The actual yield for the reaction is given and the theoretical yield can be calculated using the 1:2 stoichiometric ratio between Fe2O3 and Fe. When 1 mol of Fe2O3 is reacted with excess C, 2 mol (or 112 g) of Fe will be produced. This mass of Fe represents the theoretical yield for the reaction. Using the formula for percent yield and the values for actual yield (91.5 g) and theoretical yield (112 g), the percent yield is calculated to be 81.7%.
Correct Response: C. (Objective 0008) The percent yield for a reaction is defined as the actual yield over theoretical yield multiplied by 100%. The actual yield for the reaction is given and the theoretical yield can be calculated using the 1:2 stoichiometric ratio between upper F e sub two upper O sub three and upper F e. When 1 mol of upper F e sub two upper O sub three is reacted with excess upper C, 2 mol (or 112 g) of upper F e will be produced. This mass of upper F e represents the theoretical yield for the reaction. Using the formula for percent yield and the values for actual yield (91.5 g) and theoretical yield (112 g), the percent yield is calculated to be eight one point seven percent.
. The actual yield for the reaction is given and the theoretical yield can be calculated using the 1:2 stoichiometric ratio between Fe2O3 and Fe. When 1 mol of Fe2O3 is reacted with excess C, 2 mol (or 112 g) of Fe will be produced. This mass of Fe represents the theoretical yield for the reaction. Using the formula for percent yield and the values for actual yield (91.5 g) and theoretical yield (112 g), the percent yield is calculated to be 81.7%.
Correct Response: C. (Objective 0008) The percent yield for a reaction is defined as the actual yield over theoretical yield multiplied by 100%. The actual yield for the reaction is given and the theoretical yield can be calculated using the 1:2 stoichiometric ratio between upper F e sub two upper O sub three and upper F e. When 1 mol of upper F e sub two upper O sub three is reacted with excess upper C, 2 mol (or 112 g) of upper F e will be produced. This mass of upper F e represents the theoretical yield for the reaction. Using the formula for percent yield and the values for actual yield (91.5 g) and theoretical yield (112 g), the percent yield is calculated to be eight one point seven percent.
Question 39
39. How many sulfate ions are present in 3Al2(SO4)3?How many sulfate molecules are present in 3 capital A lowercase L subscript 2 open parenthesis capital S O subscript 4 close parenthesis subscript 3?
- 3
- 4
- 9
- 12
Answer to question 39
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0008) In this chemical formula, the coefficient 3 indicates that there are 3 molecules of Al2(SO4)3, aluminum sulfate. (SO4) represents one sulfate molecule. The subscript 3 indicates that there are three sulfate molecules in one molecule of aluminum sulfate. Therefore, the number of sulfate molecules present equals 3 × 3, or 9.
Correct Response: C. (Objective 0008) In this chemical formula, the coefficient 3 indicates that there are 3 molecules of capital A lowercase L subscript 2 open parenthesis capital S O subscript 4 close parenthesis subscript 3, aluminum sulfate. Open parenthesis capital S O subscript 4 close parenthesis represents one sulfate molecule. The subscript 3 indicates that there are three sulfate molecules in one molecule of aluminum sulfate. Therefore, the number of sulfate molecules present equals 3 times 3, or 9.
Question 40
40. Use the equation below to answer the question that follows.
PbS(s) + 4H2O2(aq) → PbSO4(s) + 4H2O(B)Capital P lowercase B capital S solid plus 4 capital H subscript 2 capital O subscript 2 aqueous yields capital P lowercase B capital S O subscript 4 solid plus 4 capital H subscript 2 capital O liquid
Which of the following number sets represents the change in oxidation number of sulfur in the reaction shown, from reactants to products?
- –2 to +6 negative 2 to positive 6
- –1 to –4negative 1 to negative 4
- +1 to –2positive 1 to negative 2
- +2 to +4positive 2 to positive 4
Answer to question 40
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0008) Finding the oxidation number of sulfur requires using the Rules for Assigning Oxidation Numbers. The oxidation state for Group 16 elements in a neutral binary compound is –2. The sum of the oxidation number in a neutral compound is 0, so the oxidation numbers of Pb and S in PbS must add to 0. Within lead sulfate (PbSO4), sulfur is within the polyatomic sulfate ion. The sulfate ion itself has a –2 total charge. Within it, each oxygen has a –2 charge. This is because oxygen has a –2 charge unless it is in a peroxide. To find sulfur's new oxidation state, first find the total charge of the oxygens in the sulfate ion: –2 * 4 = –8. Then, find the difference between this number and the sulfate ion's total charge: –8 + x = –2. Solving for x yields +6.
Correct Response: A. (Objective 0008) Finding the oxidation number of sulfur requires using the Rules for Assigning Oxidation Numbers. The oxidation state for Group 16 elements in a neutral binary compound is negative 2. The sum of the oxidation number in a neutral compound is 0, so the oxidation numbers of capital P lowercase B and capital S in capital P lowercase B capital S must add to 0. Within lead sulfate, capital P lowercase B capital S O subscript 4, sulfur is within the polyatomic sulfate ion. The sulfate ion itself has a negative 2 total charge. Within it, each oxygen has a negative 2 charge. This is because oxygen has a negative 2 charge unless it is in a peroxide. To find sulfur's new oxidation state, first find the total charge of the oxygens in the sulfate ion: negative 2 times 4 equals negative 8. Then, find the difference between this number and the sulfate ion's total charge: negative 8 + x equals negative 2. Solving for x yields positive 6.