Chemistry (Grades 9–12)
Subtest 2 Sample Items
Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
Expand All | Collapse All
Question 1
1.
| Bond |
Bond Enthalpy
(kL/mol) |
| N–N |
163 |
| N≡N |
941 |
| N–H |
391 |
| H–H |
436 |
N2(g) + 3H2(g) → 2NH3(g)
According to the information shown, what is the best estimate of the enthalpy change for this reaction?
- −1076 kJ
- −875 kJ
- −208 kJ
- −97 kJ
Answer to question 1
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0009) The enthalpy change for a reaction ( ΔHrxn) can be estimated using bond enthalpy values. The estimated value of ΔHrxn is equal to the sum of the enthalpy changes for all of the bonds that are broken in the reaction minus the sum of the enthalpy changes for all of the new bonds formed in the reaction. In this reaction, 1 mol of N≡N bonds is broken, 3 mol of H–H bonds are broken, and 6 mol of N–H bonds are formed. According to the bond enthalpy values given, the best estimate of the enthalpy change for this reaction is −97 kJ.
Correct Response: D. (Objective 0009) The enthalpy change for a reaction open parenthesis delta h sub r x n close parenthesis can be estimated using bond enthalpy values. The estimated value of delta h sub r x n is equal to the sum of the enthalpy changes for all of the bonds that are broken in the reaction minus the sum of the enthalpy changes for all of the new bonds formed in the reaction. In this reaction, 1 mol of upper N triple bond upper N bonds is broken, 3 mol of upper H single bond upper H bonds are broken, and 6 mol of upper N single bond upper H bonds are formed. According to the bond enthalpy values given, the best estimate of the enthalpy change for this reaction is negative 97 k J.
Question 2
2.
| Compound |
ΔH1°
(kJ/mol) |
| H(g) |
217.998 |
| H2(g) |
0 |
| Br(g) |
111.87 |
| Br2(g) |
30.91 |
| HBr(g) |
−36.29 |
H2(g) + Br2(g) → 2HBr(g)
According to the standard enthalpy of formation data shown, what is the standard enthalpy change for the reaction between H2(g) and Br2(g)?
- −67.20 kJnegative 67.20 kilojoules
- −72.58 kJnegative 72.58 kilojoules
- −103.49 kJnegative 103.49 kilojoules
- −394.33 kJnegative 394.33 kilojoules
Answer to question 2
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0009) The standard enthalpy of formation (ΔHf°) for a compound is the enthalpy change associated with the formation of 1 mol of the compound from its constituent elements in their standard states. The ΔHf° for an element in its standard state is zero. Standard enthalpies of formation can be used to calculate the standard enthalpy change for a reaction. The standard enthalpy change for a reaction is equal to the sum of the standard enthalpy of formation values for the products minus the sum of the standard enthalpy of formation values for the reactants (ΔHrxn° = ∑ΔHf° [products] − ∑ΔHf° [reactants]). If more than 1 mol of a product or a reactant is present, the standard enthalpy of formation value must be multiplied by the number of moles present. According to the information shown, the standard enthalpy change for the reaction between H2(g) and Br2(g) is −103.49 kJ.
Correct Response: C. (Objective 0009) The standard enthalpy of formation open parenthesis delta h sub f degrees close parenthesis for a compound is the enthalpy change associated with the formation of 1 mol of the compound from its constituent elements in their standard states. The delta h sub f degrees for an element in its standard state is zero. Standard enthalpies of formation can be used to calculate the standard enthalpy change for a reaction. The standard enthalpy change for a reaction is equal to the sum of the standard enthalpy of formation values for the products minus the sum of the standard enthalpy of formation values for the reactants open parenthesis delta h sub r x n degrees equals summation delta h sub f degrees right bracket products left bracket minus summation delta h sub f degrees left bracket reactants right bracket close parenthesis. If more than 1 mol of a product or a reactant is present, the standard enthalpy of formation value must be multiplied by the number of moles present. According to the information shown, the standard enthalpy change for the reaction between upper H sub two open parenthesis g close parenthesis and upper B r sub two open parenthesis g close parenthesis is negative 103.49 k J.
Question 3
3. Ni(s) + 2H+(aq) → Ni2+(aq) + H2(g)
A chemical equation reads as follows;
Capital N lowercase I, solid, reacts with two capital H superscript positive one, aqueous, to produce capital N lowercase I superscript positive two, aqueous, and capital H subscript two, gas.
In the reaction shown above, 2 moltwo moles of electrons are transferred to H+ and the standard electrochemical cell potential is 0.25 V.zero point two five volts Based on this information, what is the value of the standard Gibbs free energy change for this reaction?
- –12 kJ/molnegative 12 kilojoules per mole
- –24 kJ/molnegative 24 kilojoules per mole
- –48 kJ/molnegative 48 kilojoules per mole
- –220 kJ/molnegative 220 kilojoules per mole
Answer to question 3
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0010) Using the information provided, the standard Gibbs free energy change for this reaction can be calculated using the equation ΔG0 = –nFE0. In this equation, n is the number of moles of electrons transferred, F is the Faraday constant, and E0 is the electromotive force. Thus:
ΔG = –2 moles e– • 96,485 Coulombs/mole • 0.25 Joules/Coulomb
ΔG = –48,242 Joules/mol or –48 kJ/mol.
Correct Response: C. (Objective 0010) Using the information provided, the standard Gibbs free energy change for this reaction can be calculated using the equation delta G subscript 0 equals negative N times F time E subscript 0. In this equation, n is the number of moles of electrons transferred, F is the Faraday constant, and E subscript 0 is the electromotive force. Thus:
delta G equals negative 2 moles of electrons times 96 thousand 485 coulmbs per mole times 0.25 joules per coulomb
delta G equals negative 48 thousand 242 joules per mole or negative 48 kilo Joules per mole.
Question 4
4. A2(g) + 3B2(g) → 2AB3(g)
The hypothetical chemical process shown is spontaneous even though the chemical reaction represents a decrease in entropy. According to the second law of thermodynamics, which of the following must be occurring to make the overall process spontaneous?
- The reaction is in a state of equilibrium.
- The entropy of the surroundings is increasing.
- The reaction is being performed at 25°C.
- The entropy of the universe is decreasing.
Answer to question 4
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0010) The second law of thermodynamics states that the entropy of the universe increases in spontaneous processes. The entropy of the universe is the sum of the entropy of the system (in this case the reaction) and the entropy of the surroundings. In this hypothetical process, the entropy of the system is decreasing as the reaction proceeds (ΔSsystem < 0). This is because there are more possible positions and energy levels represented by the reactant molecules than by the product. In order for the overall process to be spontaneous (ΔSuniverse > 0), the entropy of the surroundings must be increasing to a greater degree than the decrease in entropy caused by the reaction.
Correct Response: B. (Objective 0010) The second law of thermodynamics states that the entropy of the universe increases in spontaneous processes. The entropy of the universe is the sum of the entropy of the system (in this case the reaction) and the entropy of the surroundings. In this hypothetical process, the entropy of the system is decreasing as the reaction proceeds open parenthesis delta s sub system less then zero close parenthesis. This is because there are more possible positions and energy levels represented by the reactant molecules than by the product. In order for the overall process to be spontaneous open parenthesis delta s sub universe greater then zero close parenthesis, the entropy of the surroundings must be increasing to a greater degree than the decrease in entropy caused by the reaction.
Question 5
5. A reaction with ΔH° = 45.0 kJ/mol and ΔS° = 72.1 J/K•mol is nonspontaneous at 298 K. Which of the following temperatures is the lowest temperature at which the reaction will be spontaneous?
- 372 K
- 451 K
- 695 K
- 804 K
Answer to question 5
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0010) The Gibbs free energy equation can be used to determine the temperature at which the given reaction will be spontaneous. The change in free energy (ΔG) for a reaction is calculated using the change in enthalpy, the change in entropy, and the temperature of the reaction (ΔG = ΔH − TΔS). Reactions that are spontaneous in the forward direction have free energy values greater than zero. According to the ΔH and ΔS values given, the temperature of this reaction must be greater than 624 K in order to be spontaneous. Therefore, of the four temperatures listed, 695 K is the lowest temperature at which the reaction will be spontaneous.
Correct Response: C. (Objective 0010) The Gibbs free energy equation can be used to determine the temperature at which the given reaction will be spontaneous. The change in free energy open parenthesis delta g close parenthesis for a reaction is calculated using the change in enthalpy, the change in entropy, and the temperature of the reaction open parenthesis delta upper g equals delta upper h minus upper t delta upper s close parenthesis. Reactions that are spontaneous in the forward direction have free energy values greater than zero. According to the delta upper h and delta upper s values given, the temperature of this reaction must be greater than 624 K in order to be spontaneous. Therefore, of the four temperatures listed, 695 K is the lowest temperature at which the reaction will be spontaneous.
Question 6
6. According to the Gibbs free energy equation, a chemical reaction is spontaneous at all temperatures under which of the following conditions?
- ΔH < 0, ΔS > 0delta H is less than 0, delta S is greater than 0
- ΔH > 0, ΔS > 0delta H is greater than 0, delta S is greater than 0
- ΔH < 0, ΔS < 0delta H is less than 0, delta S is less than 0
- ΔH > 0, ΔS < 0delta H is greater than 0, delta S is less than 0
Answer to question 6
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0010) The Gibbs equation for free energy, in which the change in free energy of a system (ΔG) is expressed in terms of changes in enthalpy (ΔH), entropy (ΔS), and temperature (T) can be used to determine the spontaneity of a chemical reaction. Specifically, use of the equation ΔG = ΔH − TΔS shows that when ΔH is negative (less than zero) and ΔS is positive (greater than zero), ΔG is always negative and the reaction is spontaneous at all temperatures.
Correct Response: A. (Objective 0010) The Gibbs equation for free energy, in which the change in free energy of a system, delta G, is expressed in terms of changes in enthalpy delta H, entropy delta S and temperature T can be used to determine the spontaneity of a chemical reaction. Specifically, use of the equation delta G equals delta H minus T multiplied by delta S shows that when delta H is negative (less than zero) and delta S is positive (greater than zero), delta G is always negative and the reaction is spontaneous at all temperatures.
Question 7
7. One mole of helium is placed in a sealed flask at 1.00 atm and 0°C. Assuming the helium in the flask behaves as an ideal gas, which of the following changes will lead to an increase in gas pressure within the flask?
- adding more helium to the flask
- decreasing the temperature to − negative 15° degrees C
- transferring the helium to a larger sealed flask
- removing one-half of the helium from the flask
Answer to question 7
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0011) Gas pressure is a measure of the force per unit area with which a gas acts upon a surface—in this case the inside of the flask. Adding more helium to the fixed volume of the flask increases the number of moles of helium present in the same volume. The presence of more helium atoms in the flask leads to more collisions between helium atoms and between helium atoms and the sides of the flask. This increase in the number of collisions results in an increase in the gas pressure within the flask.
Correct Response: A. (Objective 0011) Gas pressure is a measure of the force per unit area with which a gas acts upon a surface—in this case the inside of the flask. Adding more helium to the fixed volume of the flask increases the number of moles of helium present in the same volume. The presence of more helium atoms in the flask leads to more collisions between helium atoms and between helium atoms and the sides of the flask. This increase in the number of collisions results in an increase in the gas pressure within the flask.
Question 8
8. An ideal gas in a 1.00 L sealed vessel at 1.00 atm and 25.0°C has a mass of 1.78 g. What is the molar mass of this gas?
- 1.35 g/mol
- 3.65 g/mol
- 26.2 g/mol
- 43.5 g/mol
Answer to question 8
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0011) The following equation can be used to determine the molar mass of a gas:
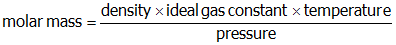 . The temperature and pressure are given for this ideal gas system and the ideal gas constant has a known value. The given temperature must be converted into kelvins before being substituted into the molar mass equation. The density of the gas can be calculated from the given mass and volume
. The temperature and pressure are given for this ideal gas system and the ideal gas constant has a known value. The given temperature must be converted into kelvins before being substituted into the molar mass equation. The density of the gas can be calculated from the given mass and volume
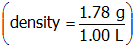 . When these values are entered into the molar mass equation, the molar mass of the gas is calculated to be 43.5 g/mol.
Correct Response: D. (Objective 0011) The following equation can be used to determine the molar mass of a gas: molar mass equals start fraction numerator density times ideal gas constant times temperature denominator pressure end fraction. The temperature and pressure are given for this ideal gas system and the ideal gas constant has a known value. The given temperature must be converted into kelvins before being substituted into the molar mass equation. The density of the gas can be calculated from the given mass and volume open parenthesis density equals start fraction numerator one point seven eight g denominator one point zero zero l end fraction close parenthesis. When these values are entered into the molar mass equation, the molar mass of the gas is calculated to be 43.5 g slash mol.
. When these values are entered into the molar mass equation, the molar mass of the gas is calculated to be 43.5 g/mol.
Correct Response: D. (Objective 0011) The following equation can be used to determine the molar mass of a gas: molar mass equals start fraction numerator density times ideal gas constant times temperature denominator pressure end fraction. The temperature and pressure are given for this ideal gas system and the ideal gas constant has a known value. The given temperature must be converted into kelvins before being substituted into the molar mass equation. The density of the gas can be calculated from the given mass and volume open parenthesis density equals start fraction numerator one point seven eight g denominator one point zero zero l end fraction close parenthesis. When these values are entered into the molar mass equation, the molar mass of the gas is calculated to be 43.5 g slash mol.
Question 9
9. If a series of different gases is allowed to effuse under the same conditions of temperature and pressure, which of the following statements correctly compares the rate of effusion of two of the gases?
- Ne will effuse one-fifth as fast as He. capital N lowercase E will effuse one fifth as fast as capital H lowercase E.
- He will effuse at the same rate as Ne.capital H lowercase E will effuse at the same rate as capital N lowercase E.
- O2 will effuse 2 times faster than H2.capital O subscript 2 will effuse 2 times faster than capital H subscript 2.
- H2 will effuse 4 times faster than O2.capital H subscript 2 will effuse 4 times faster than capital O subscript 2.
Answer to question 9
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0011) The rate of effusion is the speed at which gaseous particles separate. It is determined by a gas law related to kinetic molecular theory known as Graham's law. Since the two gases have the same temperature conditions, according to kinetic molecular theory, they must have the same average kinetic energy, which is represented as:
0.5mov2o = 0.5mHv2H
where m is mass and v is velocity. Then, to isolate the velocities on one side of the equation:
mov2o = mHv2H
mo/mH = v2H/v2o
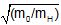 = vH/vo
Plugging in the molecular weights of O2 and H2 gives
= vH/vo
Plugging in the molecular weights of O2 and H2 gives 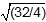 , which yields
, which yields 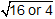 .
Correct Response: D. (Objective 0011) The rate of effusion is the speed at which gaseous particles separate. It is determined by a gas law related to kinetic molecular theory known as Graham's law. Since the two gases have the same temperature conditions, according to kinetic molecular theory, they must have the same average kinetic energy, which is represented as one-half the mass of O times the velocity of O squared equals one-half of the mass of H times the velocity of H squared. Then, to isolate the velocities on one side of the equation first get the mass of O times the velocity of O squared equals the mass of H times the velocity of H squared. Then, the mass of O divided by the mass of H equals the velocity of H squared divided by the velocity of O squared. Next, the square root open parenthesis of the mass of O divided by the mass of H close parenthesis equals the velocity of H divided by the velocity of O.
Plugging in the molecular weights of capital O subscript 2 and capital H subscript 2 gives the square root of open parenthesis 32 divided by 4 close parenthesis, which yields the square root of 16 or 4.
.
Correct Response: D. (Objective 0011) The rate of effusion is the speed at which gaseous particles separate. It is determined by a gas law related to kinetic molecular theory known as Graham's law. Since the two gases have the same temperature conditions, according to kinetic molecular theory, they must have the same average kinetic energy, which is represented as one-half the mass of O times the velocity of O squared equals one-half of the mass of H times the velocity of H squared. Then, to isolate the velocities on one side of the equation first get the mass of O times the velocity of O squared equals the mass of H times the velocity of H squared. Then, the mass of O divided by the mass of H equals the velocity of H squared divided by the velocity of O squared. Next, the square root open parenthesis of the mass of O divided by the mass of H close parenthesis equals the velocity of H divided by the velocity of O.
Plugging in the molecular weights of capital O subscript 2 and capital H subscript 2 gives the square root of open parenthesis 32 divided by 4 close parenthesis, which yields the square root of 16 or 4.
Question 10
10. Use the equation below to answer the question that follows.
C3H7OH (ℓ) + 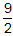 O2(g) → 3CO2(g) + 4H2O(g)capital C subscript 3 capital H subscript 7 capital O H liquid plus nine halves capital O subscript 2 gas yields 3 capital C O subscript 2 gas plus 4 capital H subscript 2 capital O gas
O2(g) → 3CO2(g) + 4H2O(g)capital C subscript 3 capital H subscript 7 capital O H liquid plus nine halves capital O subscript 2 gas yields 3 capital C O subscript 2 gas plus 4 capital H subscript 2 capital O gas
Which of the following equations represents the approximate enthalpy change for this reaction at constant temperature and pressure?
- ΔE −
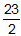 RT delta E minus twenty-three halves times R T
RT delta E minus twenty-three halves times R T
- ΔE −
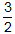 RT delta E minus three halves times R T
RT delta E minus three halves times R T
- ΔE −
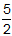 RT delta E plus five halves times R T
RT delta E plus five halves times R T
- ΔE −
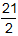 RT delta E plus twenty-one halves times R T
RT delta E plus twenty-one halves times R T
Answer to question 10
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0011) According to the first law of thermodynamics: ΔE = q + w. In a gaseous system, gases do work (w) according to –PΔV. Therefore, with the ideal gas equation, w also equals – ΔngRT, where Δng = ngas, product − ngas, reactant. Additionally, q = ΔH at constant pressure. Substituting for q and w in the first law of thermodynamics yields ΔE = ΔH − ΔngRT. Therefore, solving this equation for the reaction system given:
Δng = (3 + 4) −
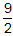 =
= 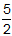 .
Plugging this back into the derived equation gives ΔH = ΔE +
.
Plugging this back into the derived equation gives ΔH = ΔE + 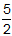 RT.
Correct Response: C. (Objective 0011) According to the first law of thermodynamics delta E equals q plus w. In a gaseous system, gases do work w according to negative P times delta V. Therefore, with the ideal gas equation, w also equals minus delta n of gas times R T, where delta n of gas equals n of gaseous product minus n of gaseous reactant. Additionally, q equals delta H at constant pressure. Substituting for q and w in the first law of thermodynamics yields delta E equals delta H minus delta n of gas times R T. Therefore, solving this equation for the reaction system given delta n of gas equals open parenthesis 3 plus 4 close parenthesis minus nine halves equals five halves. Plugging this back into the derived equation gives delta H equals delta E plus five halves times R T.
RT.
Correct Response: C. (Objective 0011) According to the first law of thermodynamics delta E equals q plus w. In a gaseous system, gases do work w according to negative P times delta V. Therefore, with the ideal gas equation, w also equals minus delta n of gas times R T, where delta n of gas equals n of gaseous product minus n of gaseous reactant. Additionally, q equals delta H at constant pressure. Substituting for q and w in the first law of thermodynamics yields delta E equals delta H minus delta n of gas times R T. Therefore, solving this equation for the reaction system given delta n of gas equals open parenthesis 3 plus 4 close parenthesis minus nine halves equals five halves. Plugging this back into the derived equation gives delta H equals delta E plus five halves times R T.
Question 11
11. The widely different melting points of CaCl2 and CH4capital C lowercase A capital C lowercase L subscript two and capital C capital H subscript four shown in the table below can be attributed to which of the following differences between the two compounds?
There is a table listing the melting points of two compounds. The first compound has the chemical formula capital C lowercase A capital C lowercase L subscript two. Its melting point is seven hundred seventy-five degrees Celsius. The second compound has the chemical formula capital C capital H subscript four. Its melting point is negative one hundred eighty-two point five degrees Celsius.
| Compound |
Melting Point (°C) |
| CaCl2capital C lowercase A capital C lowercase L subscript two |
775 |
| CH4capital C capital H subscript four |
–182.5negative one hundred eighty-two point five |
- The molar mass of CaCl2capital C lowercase A Capital C lowercase L subscript two is greater than the molar mass of CH4capital C capital H subscript four.
- The two compounds are in different states at standard temperature and pressure conditions.
- The two compounds have a different number of valence electrons.
- The attractive forces between CH4capital C capital H subscript four molecules are weaker than the attractive forces present between the ions that form CaCl2capital C lowercase A Capital C lowercase L subscript two.
Answer to question 11
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0012) In general, the increase in the melting point of substances corresponds to the increase of strength of the intermolecular forces in the substance. Calcium chloride (CaCl2) is an ionic compound, and the high melting point associated with this substance is a reflection of the large amount of energy needed to break the ionic bonds holding the atoms together within a crystalline lattice. In the case of methane (CH4), covalent bonds hold the atoms together within the molecule, but much weaker forces hold the molecules together so that much less energy is needed for melting to take place.
Correct Response: D. (Objective 0012) In general, the increase in the melting point of substances corresponds to the increase of strength of the intermolecular forces in the substance. Calcium chloride (capital C lowercase A Capital C lowercase L subscript two) is an ionic compound, and the high melting point associated with this substance is a reflection of the large amount of energy needed to break the ionic bonds holding the atoms together within a crystalline lattice. In the case of methane (capital C capital H subscript four), covalent bonds hold the atoms together within the molecule, but much weaker forces hold the molecules together so that much less energy is needed for melting to take place.
Question 12
12.
Chemical
Interaction |
Characteristic |
| Ionic bond |
bond formed by electrostatic attraction between the ions of the bonding atoms |
| Metallic bond |
valance electrons move freely throughout the crystal structure |
| Polar covalent |
bonding pair electrons are attracted to one atom more than the other atom |
| Ion-dipole forces |
attractive force present between an ion and a polar molecule |
| Dipole-dipole forces |
|
Which of the following statements will complete the table of chemical interaction characteristics shown?
- attractive force present between a Group 2 atom and a halogen
- attractive force present between atoms with expanded octets
- attractive force present between two polar molecules
- attractive force present between molecules with high molar masses
Answer to question 12
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0012) Dipole–dipole forces exist between two polar molecules. They arise because polar molecules have a permanent dipole—one end of the molecule has a slight negative charge and one end has a slight positive charge. When two polar molecules encounter one another, the positive end of one molecule is attracted to the negative end of the other molecule through electrostatic forces.
Correct Response: C. (Objective 0012) Dipole–dipole forces exist between two polar molecules. They arise because polar molecules have a permanent dipole—one end of the molecule has a slight negative charge and one end has a slight positive charge. When two polar molecules encounter one another, the positive end of one molecule is attracted to the negative end of the other molecule through electrostatic forces.
Question 13
13. C–N < C–O < C–F
Question 13
The three carbon-containing bonds shown above are arranged in order of increasing bond strength, with C–F being the strongest of the bonds listed. Which of the following factors contributes to this trend in bond strength?
- the availability of d-type electron orbitals in the C–O and C–F bonds
- a greater difference in the electronegativity values of the two bonding atoms
- the presence of more bonding electrons in the C–O and C–F bonds
- a significant increase in the length of the bonds between the two bonding atoms
Answer to question 13
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0012) Electronegativity is a measure of the degree to which atoms attract electrons in a chemical bond. When one bonding atom attracts the bonding electron(s) to a greater degree than the other, that atom will acquire a slight negative charge and the other bonding atom will acquire a slight positive charge. This difference in charge between the two bonding atoms produces a slight coulombic (or electrostatic) force between them, which contributes to the overall strength of the bond. In this example, the difference in electronegativity between C and F is greater than the electronegativity differences between C and the other two bonding atoms.
Correct Response: B. (Objective 0012) Electronegativity is a measure of the degree to which atoms attract electrons in a chemical bond. When one bonding atom attracts the bonding electron(s) to a greater degree than the other, that atom will acquire a slight negative charge and the other bonding atom will acquire a slight positive charge. This difference in charge between the two bonding atoms produces a slight coulombic (or electrostatic) force between them, which contributes to the overall strength of the bond. In this example, the difference in electronegativity between C and F is greater than the electronegativity differences between C and the other two bonding atoms.
Question 14
14. Diamond and graphite are two allotropes of carbon. The molecular characteristics of these substances cause them to have different physical properties. For instance, the fact that graphite conducts electricity while diamond does not is due to the fact that, compared to diamond, graphite has:
- a more layered structure.
- more sp3 hybridized atoms.
- a more extensive covalent bonding network.
- more delocalized pi electrons.
Answer to question 14
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0012) The carbon atoms in diamond are sp3 hybridized, and all the valence electrons are in a bound state. In graphite, however, the carbon atoms are sp2 hybridized, which means that each carbon atom has an unbound, mobile valence electron. This mobile valence electron is what allows for graphite to conduct electricity while diamond cannot.
Correct Response: D. (Objective 0012) The carbon atoms in diamond are S P 3 hybridized and all the valence electrons are in a bound state. In graphite, however, the carbon atoms are S P 2 hybridized, which means that each carbon atom has an unbound, mobile valence electron. This mobile valence electron is what allows for graphite to conduct electricity while diamond cannot.
Question 15
15. A materials engineer is hired to develop a polymer that uses ethylene as the starting material. This polymer must meet the needs of a client who requires a single polymer for a car battery casing, a microwavable food storage container, and the insulation for a high-voltage electrical wire. To meet the needs of the client, the materials engineer would most appropriately focus on developing a polymer that keeps chain-branching:
- high and uses relatively short polymer chains.
- low and uses more atoms in each cross-link that is formed between polymer chains.
- high and uses polymer chains with a low degree of crystallinity.
- low and uses polymer chains with relatively high molecular weight side groups.
Answer to question 15
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0012) A polymer for the products listed would need to be strong, but not brittle. It would also need to have some degree of thermal and chemical stability. The use of higher molecular weight side groups stiffens the polymer due to their bulk, reducing the polymer's flexibility. Other characteristics that could be used for the polymer that would produce flexible yet strong material include low chain-branching, high crystallinity, and short cross-linking chains. Together, these characteristics produce a high-density, high-melting-point, and relatively durable polymer.
Correct Response: D. (Objective 0012) A polymer for the products listed would need to be strong, but not brittle. It would also need to have some degree of thermal and chemical stability. The use of higher molecular weight side groups stiffens the polymer due to their bulk, reducing the polymer's flexibility. Other characteristics that could be used for the polymer that would produce flexible yet strong material include low chain-branching, high crystallinity, and short cross-linking chains. Together, these characteristics produce a high-density, high-melting-point, and relatively durable polymer.
Question 16
16. Use the passage and diagram below to answer the question that follows.
One possible way to identify an unknown atom in a molecule is to measure bond energy within the molecule while increasing the internuclear distance between the atoms. In the diagram, the bond energy between H and the atoms X, Y, and Z in their respective molecules are shown with increasing molecular distance.
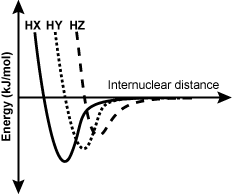 There are three lines shown, labeled from left to right as capital H capital X, capital H capital Y, and capital H capital Z. Each line starts with the same amount of energy but with increasing molecular distance. All lines dip to a low amount of energy then rise to the same middle amount of energy. However, capital H capital X dips the farthest, capital H capital Y doesn't dip quite as far, and capital H capital Z dips the least.
There are three lines shown, labeled from left to right as capital H capital X, capital H capital Y, and capital H capital Z. Each line starts with the same amount of energy but with increasing molecular distance. All lines dip to a low amount of energy then rise to the same middle amount of energy. However, capital H capital X dips the farthest, capital H capital Y doesn't dip quite as far, and capital H capital Z dips the least.
Based on the information in the diagram, which set of molecules correctly identifies HX, HY capital H capital X, capital H capital Y , and HZ capital H capital Z , respectively?
- HBr, HCl, and HIcapital H capital B lowercase R, then capital H capital C lowercase L, and then capital H capital I
- HCl, HBr, and HIcapital H capital C lowercase L, then capital H capital B lowercase R, and then capital H capital I
- HI, HCl, and HBrcapital H capital I, then capital H capital C lowercase L, and then capital H capital B lowercase R
- HBr, HI, and HClcapital H capital B lowercase R, then capital H capital I, and then capital H capital C lowercase L
Answer to question 16
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0012) The strength of a bond is measured by its dissociation energy. According to the graph, bond strength weakens with increasing internuclear distance. Increasing internuclear distance, which can also be expressed as bond length, is due to the size of the bonded atoms. Since the size of hydrogen is kept constant in each molecule, then the size of the atoms in the molecules given increases in the order X < Y < Z, which corresponds to the increasing size of the halogen elements, Cl < Br < I.
Correct Response: B. (Objective 0012) The strength of a bond is measured by its dissociation energy. According to the graph, bond strength weakens with increasing internuclear distance. Increasing internuclear distance, which can also be expressed as bond length is due to the size of the bonded atoms. Since the size of hydrogen is kept constant in each molecule, then the size of the atoms in the molecules given increases in the order X is less than Y is less than Z, which corresponds to the increasing size of the halogens, capital C lowercase L is less than capital B lowercase R is less than capital I.
Question 17
17. Which of the following compounds has a linear molecular geometry?
- SCN–The chemical formula for the compound is capital S capital C capital N superscript negative one.
- BrF5 The chemical formula for the compound is capital B lowercase R capital F subscript five.
- NO
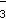 The chemical formula for the compound is capital N capital O superscript negative one subscript three.
The chemical formula for the compound is capital N capital O superscript negative one subscript three.
- H3O+ The chemical formula for the compound is capital H subscript three capital O superscript positive one.
Answer to question 17
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0013) According to the valence-shell electron-pair repulsion (VSPER) model, the geometrical arrangement between atoms is such that the repulsion arising from the electron domains is minimized. Based on the Lewis structure for SCN–, there are a number of resonance structures that allow a complete octet around each element. In each case, carbon is the central atom and is bonded to sulfur on one side and nitrogen on the other. There are two electron bonding domains in this ion, and the resulting molecular geometry is thus linear.
Correct Response: A. (Objective 0013) According to the valence-shell electron-pair repulsion (V S P E R) model, the geometrical arrangement between atoms is such that the repulsion arising from the electron domains is minimized. Based on the Lewis structure for capital S capital C capital N superscript negative one, there are a number of resonance structures that allow a complete octet around each element. In each case, carbon is the central atom and is bonded to sulfur on one side and nitrogen on the other. There are two electron bonding domains in this ion, and the resulting molecular geometry is thus linear.
Question 18
18. Which of the following is the chemical formula for iron(III) oxide?
- Fe3O
- Fe2O3
- FeO3
- Fe3O2
Answer to question 18
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0013) According to IUPAC rules of nomenclature, the chemical formula for iron(III) oxide is Fe2O3. The name of the compound indicates that the iron (Fe) in this compound has an oxidation number of 3. The other atom in the compound, oxygen (O), usually has an oxidation number of −2. Iron(III) oxide is a neutral compound. This means that the sum of the oxidation numbers for all of the Fe and O atoms must equal zero. When 2 Fe atoms are combined with 3 O atoms, the oxidation numbers are 6 and −6, and the compound is neutral.
Correct Response: B. (Objective 0013) According to I U P A C rules of nomenclature, the chemical formula for iron (III) oxide is upper F e sub two upper O sub three. The name of the compound indicates that the iron (upper F e) in this compound has an oxidation number of 3. The other atom in the compound, oxygen (upper O), usually has an oxidation number of negative 2. Iron (III) oxide is a neutral compound. This means that the sum of the oxidation numbers for all of the upper F e and upper O atoms must equal zero. When 2 upper F e atoms are combined with 3 upper O atoms, the oxidation numbers are 6 and negative 6, and the compound is neutral.
Question 19
19. A student observes that a sample of solid NaCl does not conduct electricity, but an aqueous solution of NaCl does conduct electricity. Which of the following best explains this difference in electrical conductivity?
- The structure of ionic crystals is too irregular.
- The crystal is held together by strong covalent bonds.
- The ions in ionic crystals are in fixed positions.
- The distance between lattice points in the crystal is too great.
Answer to question 19
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0013) NaCl is an ionic solid composed of Na+ ions and Cl− ions. In its solid form, the Na+ ions and Cl− ions are held in fixed positions in the crystal structure. This lack of movement by the ions within the crystal structure prevents the conduction of electricity.
Correct Response: C. (Objective 0013) upper N a upper C l is an ionic solid composed of upper N a positive ions and upper C l negative ions. In its solid form, the upper N a positive ions and upper C l negative ions are held in fixed positions in the crystal structure. This lack of movement by the ions within the crystal structure prevents the conduction of electricity.
Question 20
20. Which of the following best describes the molecular geometry of PF5?
- trigonal bipyramidal
- linear
- trigonal planar
- tetrahedral
Answer to question 20
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0013) The valence-shell electron-pair repulsion theory can be used to predict the molecular geometry of PF5. The first step in this process is determining the Lewis structure of PF5. From the Lewis structure of PF5, it is known that there are five electron bonding pairs surrounding the central P atom. Compounds in which the central atom has five electron bonding pairs and no lone pairs exhibit trigonal bipyramidal geometry.
Correct Response: A. (Objective 0013) The valence-shell electron-pair repulsion theory can be used to predict the molecular geometry of upper P upper F sub five. The first step in this process is determining the Lewis structure of upper P upper F sub five. From the Lewis structure of upper P upper F sub five, it is known that there are five electron bonding pairs surrounding the central upper P atom. Compounds in which the central atom has five electron bonding pairs and no lone pairs exhibit trigonal bipyramidal geometry.
Question 21
21.
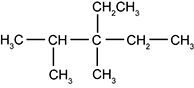
On the second carbon, there is a singly bonded CH sub 3 group, and on the third carbon, there is a single bonded C H sub 3 group as well as a singly bonded C H sub 2 C H sub 3 group.
What is the IUPAC name for the molecule shown above?
- 2-methyl-3,3-diethylpropane
- 3-ethyl-2,3-dimethylpentane
- 2,3-diethyl-3-methylpropene
- 3-methyl-3,4-diethylpentene
Answer to question 21
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0014) The IUPAC name of the given molecule can be determined by analyzing its chemical structure. The name of the hydrocarbon shown is based on the length of the longest continuous chain of carbon atoms in the molecule. In this molecule the longest continuous chain consists of five carbon atoms and all the carbons are bonded with single bonds. This information identifies the molecule as a pentane. Three alkyl groups are attached at carbons 2 and 3 of this molecule. Two of the side groups are methyl groups (CH3) and one is an ethyl group (CH2CH3). Both the type of alkyl group and its location must be included in the name of the molecule. The numbering of C atoms begins at the end of the molecule closest to a side group. The two CH3 side groups are attached at carbons 2 and 3, and the CH2CH3 side group is attached at carbon 3. When two different types of side groups are present, the side groups are listed in alphabetical order. Given the information shown in the chemical structure, this molecule is 3-ethyl-2,3-dimethylpentane.
Correct Response: B. (Objective 0014) The I U P A C name of the given molecule can be determined by analyzing its chemical structure. The name of the hydrocarbon shown is based on the length of the longest continuous chain of carbon atoms in the molecule. In this molecule the longest continuous chain consists of five carbon atoms and all the carbons are bonded with single bonds. This information identifies the molecule as a pentane. Three alkyl groups are attached at carbons 2 and 3 of this molecule. Two of the side groups are methyl groups open parenthesis upper c upper h sub three and one is an ethyl group open parenthesis upper c upper h sub two upper c upper h sub three close parenthesis. Both the type of alkyl group and its location must be included in the name of the molecule. The numbering of upper C atoms begins at the end of the molecule closest to a side group. The two upper C upper H sub three side groups are attached at carbons 2 and 3, and the upper C upper H sub two upper C upper H sub three side group is attached at carbon 3. When two different types of side groups are present, the side groups are listed in alphabetical order. Given the information shown in the chemical structure, this molecule is 3-ethyl-2,3-dimethylpentane.
Question 22
22.
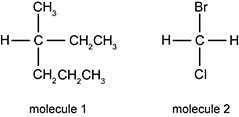
Molecule 1 has a central carbon with four singly bonded groups: upper C upper H sub three, a upper C upper H sub two upper C upper H sub three, a upper C upper H sub two upper C upper H sub 2 upper C upper H sub 3, and a hydrogen clockwise around the carbon center. Molecule 2 has a central carbon with four singly bonded groups: a Bromine, a hydrogen, a Chlorine, and another hydrogen clockwise around the carbon center.
When the two molecules shown above were tested in a polarimeter, only molecule 1 was found to rotate polarized light. The lack of optical activity observed with molecule 2 can be attributed to which of the following molecular characteristics?
- Molecule 2 lacks an ethyl side group (CH2CH3).
- The molecular weight of molecule 2 is too low.
- olecule 2 has halogen atoms bonded to its central C atom.
- The mirror images of molecule 2 are superimposable.
Answer to question 22
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0014) Optical activity is a characteristic displayed by chiral molecules—compounds with nonsuperimposable mirror images. Chiral molecules often possess a central carbon atom that is bonded to four different atoms or groups of atoms. In the given molecules, molecule 1 has a central carbon atom that is bonded to four different groups of atoms and it displays optical activity. Molecule 2 lacks this type of bonding arrangement around its central carbon atom. As a result, its mirror images are superimposable and it does not display optical activity.
Correct Response: D. (Objective 0014) Optical activity is a characteristic displayed by chiral molecules—compounds with nonsuperimposable mirror images. Chiral molecules often possess a central carbon atom that is bonded to four different atoms or groups of atoms. In the given molecules, molecule 1 has a central carbon atom that is bonded to four different groups of atoms and it displays optical activity. Molecule 2 lacks this type of bonding arrangement around its central carbon atom. As a result, its mirror images are superimposable and it does not display optical activity.cal order. Given the information shown in the chemical structure, this molecule is 3-ethyl-2,3-dimethylpentane.
Question 23
23. Which of the following techniques would most effectively determine the structure of a protein?
- thin-layer chromatography
- infrared spectroscopy
- gas chromatography
- ultraviolet spectroscopy
Answer to question 23
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0014) Thin-layer chromatography (TLC) is one of a number of methods to determine the structure of a protein and is, in general, a method that works best to identify solid nonvolatile organic compounds of a range of molecular sizes. In TLC, there is a stationary phase and a mobile phase of solvent. The sample is placed at one end of the stationary phase. The mobile phase then wicks onto the stationary phase behind the sample and moves the sample along the stationary phase until the sample falls out of solution. In this case, the protein would be split into peptides before the sample was run. As the sample ran, the peptide groups would separate based on size and hydrophobicity.
Correct Response: A. (Objective 0014) Thin-layer chromatography T L C is one of a number of methods to determine the structure of a protein and is, in general, a method that works best to identify solid nonvolatile organic compounds of a range of molecular sizes. In T L C, there is a stationary phase and a mobile phase of solvent. The sample is placed at one end of the stationary phase. The mobile phase then wicks onto the stationary phase behind the sample and moves the sample along the stationary phase until the sample falls out of solution. In this case, the protein would be split into peptides before the sample was run. As the sample ran, the peptide groups would separate based on size and hydrophobicity.
Question 24
24. HF(aq) + KOH(aq) → KF(aq) + H2O(ℓ)
The equation shown above is an example of which of the following types of chemical reactions?
- precipitation
- acid-base
- single replacement
- combustion
Answer to question 24
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0015) This is an example of an acid-base reaction. In this reaction, HF is a Brønsted acid (a proton donor). The proton (H+) from HF reacts with the hydroxide ion (OH−) from KOH to produce a salt (KF) and H2O.
Correct Response: B. (Objective 0015) This is an example of an acid-base reaction. In this reaction, upper H upper F is a Brønsted acid (a proton donor). The proton open parenthesis upper h positive close parenthesis from upper H upper F reacts with the hydroxide ion open parenthesis upper o upper h negative close parenthesis from upper K upper O upper H to produce a salt open parenthesis upper K upper F close parenthesis and upper H sub two upper O.
Question 25
25.
| Metal |
Reducing
Strength |
| Li |
Strongest
↑
Weakest |
| Zn |
| Cu |
| Hg |
| Pt |
| Au |
Of the six metals shown in the activity series above, Li is the most easily oxidized and Au is the least easily oxidized. According to the information in the table, which of the following metals in its elemental state will displace Cu ions from aqueous CuSO4upper C u upper S upper O subscript four?
- Zn
- Hg
- Pt
- Au
Answer to question 25
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0015) The information in the activity series table can be used to predict the outcome of a reaction between a metal and aqueous CuSO4. The table lists Li as the metal most easily oxidized (loses electrons), Au as the metal least easily oxidized, and Cu in the middle of the range. Metals that are more easily oxidized than Cu are more reactive and will displace Cu ions from aqueous CuSO4. Of the metals listed, Zn is the metal that will displace Cu from aqueous CuSO4.
Correct Response: A. (Objective 0015) The information in the activity series table can be used to predict the outcome of a reaction between a metal and aqueous upper C u upper S upper O sub four. The table lists upper L i as the metal most easily oxidized (loses electrons), upper A u as the metal least easily oxidized, and upper C u in the middle of the range. Metals that are more easily oxidized than upper C u are more reactive and will displace upper C u ions from aqueous upper C u upper S upper O sub four. Of the metals listed, upper Z n is the metal that will displace upper C u from aqueous upper C u upper S upper O sub four.
Question 26
26. Which of the following is a product formed from the decomposition of methanol (CH3OH)?
- H2O(ℓ)
- CO2(g)
- OH−negative(aq)
- H2(g)
Answer to question 26
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0015) The decomposition of CH3OH will produce H2 and CO. The balanced equation for this decomposition reaction is CH3OH(ℓ) → 2H2(g) + CO(g).
Correct Response: D. (Objective 0015) The decomposition of upper C upper H sub three upper O upper H will produce upper H sub two and upper C upper O. The balanced equation for this decomposition reaction is upper C upper H sub three upper O upper H open parenthesis ℓ close parenthesist produces two upper h sub two open parenthesis g close parenthesis plus upper c upper o open parenthesis g close parenthesis.
Question 27
27. Use the equation below to answer the question that follows.
Ba2+(aq) + 2OH–(aq) + 2H+(aq) + SO42–(aq) → BaSO4(s) + 2H2O(ℓ)capital B lowercase A superscript positive 2 aqueous plus 2 capital O H superscript negative one aqueous plus 2 capital H superscript positive 1 aqueous plus capital S O subscript 4 superscript negative 2 aqueous yields capital B lowercase A capital S O subscript 4 solid plus 2 capital H subscript 2 capital O liquid
In order to demonstrate the conductivity of acids and bases, a teacher performs a conductometric titration of barium hydroxide with sulfuric acid. The equation for this reaction is shown. Which of the following descriptions of this process is most accurate?
- One reaction occurred: a neutralization reaction.
- Two reactions occurred: a neutralization and a precipitation reaction.
- Three reactions occurred: a neutralization, a redox, and a precipitation reaction.
- Four reactions occurred: a neutralization, a precipitation, a redox, and a substitution reaction.
Answer to question 27
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0015) Two reactions occurred simultaneously in the demonstration. First, an insoluble salt was formed in a precipitation reaction in which Ba2+(aq) + SO42–(aq) → BaSO4(s). Additionally, acidic and basic ions combined to form a neutral molecule in a neutralization reaction in which 2H+(aq) + 2OH–(aq) → 2H2O(ℓ).
Correct Response: B. (Objective 0015) Two reactions occurred simultaneously in the demonstration. First, an insoluble salt was formed in a precipitation reaction in which capital B lowercase A superscript positive 2 aqueous plus capital S O subscript 4 superscript negative 2 aqueous yields capital B lowercase A capital S O subscript 4 solid. Additionally, acidic and basic ions combined to form a neutral molecule in a neutralization reaction in which 2 capital H superscript positive 1 aqueous plus 2 capital O H superscript negative one aqueous yields 2 capital H subscript 2 capital O liquid.
Question 28
28. A chemical reaction is proposed to take place in a single elementary step. Which of the following provides the best evidence to support the proposed reaction mechanism?
- The balanced chemical equation for the reaction consists of a single reactant.
- The experimentally determined rate law is equal to the rate law consistent with a single elementary step.
- The reaction mechanism does not involve the formation of reaction intermediates.
- The rate constant for the rate law corresponding to the single elementary step is equal to one.
Answer to question 28
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0016) For all chemical reactions, the proposed reaction mechanism must meet a set of requirements that includes the fact that the experimentally determined rate law for the reaction is equal to the rate law associated with a single elementary step. In addition, the equation for the overall reaction must reflect all of the elementary reaction equations. In the case of a chemical reaction that takes place in a single elementary step, the rate law for the overall equation, therefore, must be consistent with the rate of the elementary (single step) reaction.
Correct Response: B. (Objective 0016) For all chemical reactions, the proposed reaction mechanism must meet a set of requirements that includes the fact that the experimentally determined rate law for the reaction is equal to the rate law associated with a single elementary step. In addition, the equation for the overall reaction must reflect all of the elementary reaction equations. In the case of a chemical reaction that takes place in a single elementary step, the rate law for the overall equation, therefore, must be consistent with the rate of the elementary (single step) reaction.
Question 29
29. 2NO(g) + Cl2(g) → 2NOCl(g)
In the reaction shown above, nitric oxide (NO) reacts with chlorine (Cl2) to produce nitrosyl chloride (NOCl). Which of the following changes to the reaction conditions is most likely to increase the rate of this reaction?
- increasing the total volume of the reaction mixture
- decreasing the temperature of the reaction mixture
- increasing the pressure on the reaction mixture
- decreasing the concentrations of NO and Cl2
Answer to question 29
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0016) According to collision theory, the rate of a reaction will typically increase as the frequency and intensity of collisions between reacting molecules increase. Since the reactants in this reaction are gases, increasing the pressure on the reaction mixture will lead to an increase in the concentration of the reactants. This in turn will lead to an increase in the number of collisions between reactant molecules and an increase in reaction rate.
Correct Response: C. (Objective 0016) According to collision theory, the rate of a reaction will typically increase as the frequency and intensity of collisions between reacting molecules increase. Since the reactants in this reaction are gases, increasing the pressure on the reaction mixture will lead to an increase in the concentration of the reactants. This in turn will lead to an increase in the number of collisions between reactant molecules and an increase in reaction rate.
Question 30
30.
| Experiment |
[A] (M) |
[B] (M) |
Initial Rate
(M/s) |
| 1 |
2.0 × 10−32.0 times 10 to the minus 3 |
1.0 × 10−31.0 times 10 to the minus 3 |
2.0 × 10−52.0 times 10 to the minus 5 |
| 2 |
2.0 × 10−32.0 times 10 to the minus 3 |
2.0 × 10−32.0 times 10 to the minus 3 |
2.0 × 10−52.0 times 10 to the minus 3 |
| 3 |
4.0 × 10−34.0 times 10 to the minus 3 |
1.0 × 10−31.0 times 10 to the minus 3 |
4.0 × 10−54.0 times 10 to the minus 3 |
The table above shows experimental rate data for the hypothetical reaction A(g) + 2B(g) → AB2(g). According to the information given, what is the value of the rate constant, k, for this reaction?
- 1.0 × 10−2 s−11.0 times 10 to the minus 2
- 2.0 × 10−2 s−12.0 times 10 to the minus 2
- 1.0 × 101 M−1s−11.0 times 10 exponent 1 M to the minus 1 s to the minus 1
- 1.0 × 104 M−2s−11.0 times 10 exponent 4 M to the minus 2 s to the minus 1
Answer to question 30
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0016) The general formula for the rate law for this reaction is rate = k[A]x[B]y. The values of x and y can be determined by analyzing the experimental rate data. Analysis of experiments 1 and 3 shows that doubling the concentration of A leads to a doubling of the initial rate. This indicates that x = 1. The value of y can be determined by comparing experiments 1 and 2. These experiments show that changing the concentration of B has no effect on the initial rate. Therefore, the value of y = 0. With the values of x and y known, the rate law for this reaction becomes rate = k[A]1[B]0. By rearranging this equation and substituting in rate and concentration values from any of the experiments, the value of k can be determined. Using this approach, k is calculated to be 1.0 × 10−2 s−1.
Correct Response: A. (Objective 0016) The general formula for the rate law for this reaction is rate equals k left bracket a right bracket sup x baseline left bracket b right bracket sup y. The values of x and y can be determined by analyzing the experimental rate data. Analysis of experiments 1 and 3 shows that doubling the concentration of A leads to a doubling of the initial rate. This indicates that x equals 1. The value of y can be determined by comparing experiments 1 and 2. These experiments show that changing the concentration of B has no effect on the initial rate. Therefore, the value of y equals 0. With the values of x and y known, the rate law for this reaction becomes rate equals k left bracket a right bracket sup one baseline left bracket b right bracket sup zero. By rearranging this equation and substituting in rate and concentration values from any of the experiments, the value of k can be determined. Using this approach, k is calculated to be 1.0 times 10 sup negative two baseline s sup negative one.
Question 31
31.
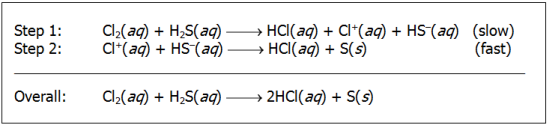
step 1: upper c l sub two open parenthesis aq close parenthesis plus upper h two upper s open parenthesis aq close parenthesis leads to upper h upper c l open parenthesis aq close parenthesis plus upper c l positive open parenthesis aq close parenthesis plus upper h upper s negative open parenthesis aq close parenthesis open parenthesis slow close parenthesis step 2: upper c l sub two open parenthesis aq close parenthesis plus upper h upper s negative open parenthesis aq close parenthesis leads to upper h upper c l open parenthesis aq close parenthesis plus upper s open parenthesis s close parenthesis Overall: Upper c l sub two open parenthesis aq close parenthesis plus upper h sub two upper s open parenthesis aq close parenthesis leads to two upper h upper c l open parenthesis aq close parenthesis plus upper s open parenthesis s close parenthesis
A chemist has proposed the reaction mechanism shown above for the reaction between Cl2 and H2S. What is the rate law for the overall reaction as described by this mechanism?
- rate = k[Cl+][HS−]
- rate = kCl2][H2S]
- rate = kHCl]2[S]
- rate = kHCl][Cl2]
Answer to question 31
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0016) The first step in the proposed mechanism occurs slowly and limits the rate at which the reaction proceeds. This is the rate-limiting step and it is the basis of the rate law for the overall reaction as described by this mechanism. The rate law for the reaction in step 1 is equal to the product of the rate constant for this step and the concentrations of the two reactants (rate = k[Cl2][H2S]). If this rate law agrees with the experimentally determined rate law for this reaction, this proposed mechanism may be the actual mechanism at work.
Correct Response: B. (Objective 0016) The first step in the proposed mechanism occurs slowly and limits the rate at which the reaction proceeds. This is the rate-limiting step and it is the basis of the rate law for the overall reaction as described by this mechanism. The rate law for the reaction in step 1 is equal to the product of the rate constant for this step and the concentrations of the two reactants open parenthesis rate equals k left bracket upper c l sub two right bracket left bracket upper h sub two upper s right bracket close parenthesis. If this rate law agrees with the experimentally determined rate law for this reaction, this proposed mechanism may be the actual mechanism at work.
Question 32
32. Use the diagram below to answer the question that follows.
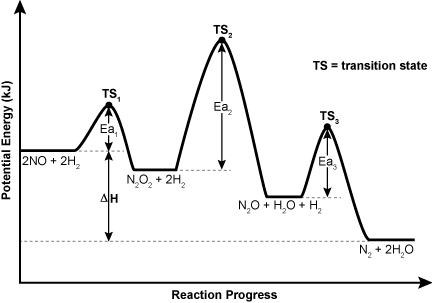 There are four steps in the diagram with a peak linking each step. Each successive step is lower on the y-axis than the last. The leftmost step is labeled 2 capital N O plus 2 capital H subscript 2. The second step is labeled capital N subscript 2 capital O subscript 2 plus 2 capital H subscript 2. The third step is labeled capital N subscript 2 capital O plus capital H subscript 2 capital O plus capital H subscript 2. The last step is labeled capital N subscript 2 plus 2 capital H subscript 2 capital O. At the maximum point on each peak is a point labeled transition point 1 on the leftmost peak, then transition point 2 and transition point 3 as the reaction progresses. The vertical distance between the transition point and the reaction step is labeled capital E lowercase A 1 then capital E lowercase A 2 and capital E lowercase A 3 as the reaction progresses. The peaks vary in height with the first peak's maximum being about half as high as the second peak's maximum. The third peak's maximum is slightly lower than the first peak's maximum. This results in the capital E lowercase A 1 being the shortest, then capital E lowercase A 3 being the middle length, and capital E lowercase A 2 being the longest. The vertical distance between the first step and the last step is labeled delta H.
There are four steps in the diagram with a peak linking each step. Each successive step is lower on the y-axis than the last. The leftmost step is labeled 2 capital N O plus 2 capital H subscript 2. The second step is labeled capital N subscript 2 capital O subscript 2 plus 2 capital H subscript 2. The third step is labeled capital N subscript 2 capital O plus capital H subscript 2 capital O plus capital H subscript 2. The last step is labeled capital N subscript 2 plus 2 capital H subscript 2 capital O. At the maximum point on each peak is a point labeled transition point 1 on the leftmost peak, then transition point 2 and transition point 3 as the reaction progresses. The vertical distance between the transition point and the reaction step is labeled capital E lowercase A 1 then capital E lowercase A 2 and capital E lowercase A 3 as the reaction progresses. The peaks vary in height with the first peak's maximum being about half as high as the second peak's maximum. The third peak's maximum is slightly lower than the first peak's maximum. This results in the capital E lowercase A 1 being the shortest, then capital E lowercase A 3 being the middle length, and capital E lowercase A 2 being the longest. The vertical distance between the first step and the last step is labeled delta H.
According to the reaction profile diagram, which pair of molecules identifies a likely activated complex and an intermediate, respectively?
- NOH2 and N2O2capital N O H subscript 2 and capital N subscript 2 capital O subscript 2
- N2OH2 and H2capital N subscript 2 capital O H subscript 2 and capital H subscript 2
- NOH2 and N2capital N O H subscript 2 and capital N subscript 2
- N2O2H4 and N2Ocapital N subscript 2 capital O subscript 2 capital H subscript 4 and capital N subscript 2 capital O
Answer to question 32
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0016) The activated complexes are the chemical structures that appear at the maximum potential energy in the reaction paths while the intermediates are more stable compounds that appear before the entire reaction path is completed. Therefore, according to the mechanism given, the main reaction intermediates are N2O2 and N2O. The possible activated complexes are: (1) H2- - -N2O2, (2) NO- - -H2, (3) N2O- - -H2, (4) H2- - -N2O2- - - H2, and (5) H2- - -NO- - -NO- - -H2. Activated complexes (4) and (5) are unlikely as they suggest the simultaneous collision of three or more molecules. Activated complexes (1), (2), and (3) are all likely because their atoms can stoichiometrically reorganize to give the products in the overall reaction.
Correct Response: A. (Objective 0016) The activated complexes are the chemical structures that appear at the maximum potential energy in the reaction paths while the intermediates are more stable compounds that appear before the entire reaction path is completed. Therefore, according to the mechanism given, the main reaction intermediates are capital N subscript 2 capital O subscript 2 and capital N subscript 2 capital O. The possible activated complexes are: (1) capital H subscript 2 in a complex with capital N subscript 2 capital O subscript 2, (2) capital N O in a complex with capital H subscript 2, (3) capital N subscript 2 capital O in a complex with capital H subscript 2, (4) capital H subscript 2 in a complex with capital N subscript 2 capital O subscript 2 and with capital H subscript 2, and (5) capital H subscript 2 in a complex with capital N O and with capital N O and with capital H subscript 2. Activated complexes (4) and (5) are unlikely as they suggest the simultaneous collision of three or more molecules. Activated complexes (1), (2), and (3) are all likely because their atoms can stoichiometrically reorganize to give the products in the overall reaction.
Question 33
33. CH4(g) + H2O(g)
 CO(g) + 3H2(g) ΔH° > 0upper C upper H sub 4 open parenthesis g closed parenthesis plus upper H sub two upper O open parenthesis g closed parenthesis rightwards harpoon over leftwards harpoon upper C upper O open parenthesis g closed parenthesis plus three upper H sub two open parenthesis g closed parenthesis delta H degrees greater than zero
CO(g) + 3H2(g) ΔH° > 0upper C upper H sub 4 open parenthesis g closed parenthesis plus upper H sub two upper O open parenthesis g closed parenthesis rightwards harpoon over leftwards harpoon upper C upper O open parenthesis g closed parenthesis plus three upper H sub two open parenthesis g closed parenthesis delta H degrees greater than zero
Which of the following changes to the equilibrium system shown above will lead to an increase in the concentration of H2H sub 2?
- adding heat to the system
- decreasing the concentration of CH4CH sub 4
- increasing the concentration of COC O
- decreasing the volume of the system
Answer to question 33
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0017) In the equilibrium system shown, H2 is a product of the forward reaction. In order to increase the concentration of H2, the change to the system must lead to a shifting of the equilibrium position in the forward direction (to the right). This equilibrium system is endothermic (ΔH° > 0) and heat added to the system will act like an increase in a reactant. In order to reestablish equilibrium, the system will use up the added heat, which will cause more H2 and CO to be produced.
Correct Response: A. (Objective 0017) In the equilibrium system shown, H sub 2 is a product of the forward reaction. In order to increase the concentration of H sub 2, the change to the system must lead to a shifting of the equilibrium position in the forward direction open parenthesis to the right closed parenthesis. This equilibrium system is endothermic open parenthesis delta h degrees greater than 0 closed parenthesis and heat added to the system will act like an increase in a reactant. In order to reestablish equilibrium, the system will use up the added heat, which will cause more upper H sub 2 and C O to be produced.
Question 34
34. CH3COOH(aq)
 CH3COO−(aq) + H+(aq) Ka = 1.8 × 10−5 upper C upper H sub 3 upper C upper O upper O upper H open parenthesis a q closed parenthesis rightwards harpoon over leftwards harpoon upper C upper H sub 3 upper C upper O upper O sup negative open parenthesis a q closed parenthesis plus upper H sup plus open parenthesis a q closed parenthesis space K sub a equals one point eight times 10 to the minus five.
CH3COO−(aq) + H+(aq) Ka = 1.8 × 10−5 upper C upper H sub 3 upper C upper O upper O upper H open parenthesis a q closed parenthesis rightwards harpoon over leftwards harpoon upper C upper H sub 3 upper C upper O upper O sup negative open parenthesis a q closed parenthesis plus upper H sup plus open parenthesis a q closed parenthesis space K sub a equals one point eight times 10 to the minus five.
Acetic acid (CH3COOH)open parenthesis upper C upper H sub 3 upper C upper O upper O upper H closed parenthesis is a weak acid that dissociates in water as shown above. Given this dissociation equation and KaK sub a of CH3COOHupper C upper H sub 3 upper C upper O upper O upper H, what is the pH of a 0.50 MCH3COOHzero point five zero M upper C upper H sub 3 upper C upper O upper O upper H solution at 25°C?
- 1.8
- 2.5
- 3.6
- 4.5
Answer to question 34
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: B. (Objective 0017) The pH of a solution can be calculated using the following formula: pH = −log[H+]. The first step in answering this question is to determine the concentration of H+ in solution at equilibrium. The [H+] can be calculated using the given Ka value for CH3COOH and the equilibrium expression for this reaction
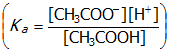 . Algebraic terms for the concentrations can be derived by tabulating the initial, change, and equilibrium concentrations for the substances in solution at equilibrium. When these terms are substituted into the equilibrium expression, a value for the [H+] can be calculated
. Algebraic terms for the concentrations can be derived by tabulating the initial, change, and equilibrium concentrations for the substances in solution at equilibrium. When these terms are substituted into the equilibrium expression, a value for the [H+] can be calculated
 . The mathematical term for the [CH3COOH] can be simplified to 0.50 because CH3COOH is a weak electrolyte and the [CH3COOH] at equilibrium is only slightly less than 0.50 M. When this equation is solved for x, the [H+] is determined to equal 3.0 × 10−3M. The final step in answering this question is to calculate the pH of the 0.50 MCH3COOH solution. This is done by substituting 3.0 × 10−3 M into the formula for pH (pH = 2.5).
Correct Response: B. (Objective 0017) The pH of a solution can be calculated using the following formula: pH = minus log left bracket upper H sup plus right bracket. The first step in answering this question is to determine the concentration of H sup + in solution at equilibrium. The left bracket upper H sup plus right bracket can be calculated using the given K sub a value for upper C upper H sub 3 upper C upper O upper O upper H and the equilibrium expression for this reaction open parenthesis K sub a equals start fraction numerator left bracket upper C upper H sub 3 upper C upper O upper O sup negative left bracket right bracket upper H sup plus right bracket denominator left bracket upper C upper H sub 3 upper C upper O upper O upper H right bracket end fraction closed paranthesis. Algebraic terms for the concentrations can be derived by tabulating the initial, change, and equilibrium concentrations for the substances in solution at equilibrium. When these terms are substituted into the equilibrium expression, a value for the left bracket upper H sup plus right bracket can be calculated open parenthesis 1.8 times 10 to the minus 5 equals start fraction numerator x bullet x denominator zero dot five zero minus x end fraction closed paranthesis. The mathematical term for the left bracket upper C upper H sub 3 upper C upper O upper O upper H right bracket can be simplified to 0.50 because upper C upper H sub 3 upper C upper O upper O upper H is a weak electrolyte and the left bracket upper C upper H sub 3 upper C upper O upper O upper H right bracket at equilibrium is only slightly less than 0.50 M. When this equation is solved for x, the left bracket upper H sup plus right bracket is determined to equal 3.0 times 10 sup negative 3 M. The final step in answering this question is to calculate the pH of the 0.50 M upper C upper H sub 3 upper C upper O upper O upper H solution. This is done by substituting 3.0 times 10 to the negative third M into the formula for pH open parenthesis pH = two point five closed parenthesis.
. The mathematical term for the [CH3COOH] can be simplified to 0.50 because CH3COOH is a weak electrolyte and the [CH3COOH] at equilibrium is only slightly less than 0.50 M. When this equation is solved for x, the [H+] is determined to equal 3.0 × 10−3M. The final step in answering this question is to calculate the pH of the 0.50 MCH3COOH solution. This is done by substituting 3.0 × 10−3 M into the formula for pH (pH = 2.5).
Correct Response: B. (Objective 0017) The pH of a solution can be calculated using the following formula: pH = minus log left bracket upper H sup plus right bracket. The first step in answering this question is to determine the concentration of H sup + in solution at equilibrium. The left bracket upper H sup plus right bracket can be calculated using the given K sub a value for upper C upper H sub 3 upper C upper O upper O upper H and the equilibrium expression for this reaction open parenthesis K sub a equals start fraction numerator left bracket upper C upper H sub 3 upper C upper O upper O sup negative left bracket right bracket upper H sup plus right bracket denominator left bracket upper C upper H sub 3 upper C upper O upper O upper H right bracket end fraction closed paranthesis. Algebraic terms for the concentrations can be derived by tabulating the initial, change, and equilibrium concentrations for the substances in solution at equilibrium. When these terms are substituted into the equilibrium expression, a value for the left bracket upper H sup plus right bracket can be calculated open parenthesis 1.8 times 10 to the minus 5 equals start fraction numerator x bullet x denominator zero dot five zero minus x end fraction closed paranthesis. The mathematical term for the left bracket upper C upper H sub 3 upper C upper O upper O upper H right bracket can be simplified to 0.50 because upper C upper H sub 3 upper C upper O upper O upper H is a weak electrolyte and the left bracket upper C upper H sub 3 upper C upper O upper O upper H right bracket at equilibrium is only slightly less than 0.50 M. When this equation is solved for x, the left bracket upper H sup plus right bracket is determined to equal 3.0 times 10 sup negative 3 M. The final step in answering this question is to calculate the pH of the 0.50 M upper C upper H sub 3 upper C upper O upper O upper H solution. This is done by substituting 3.0 times 10 to the negative third M into the formula for pH open parenthesis pH = two point five closed parenthesis.
Question 35
35. CaSO4(s)
 Ca2+(aq) + SO42−(aq) ΔG° = 26.3 kJ/mol upper C a upper S upper O sub 4 open parenthesis s closed parenthesis rightwards harpoon over leftwards harpoon upper C a sup 2 plus open parenthesis aq closed parenthesis plus upper S upper O sub 4 sup 2 negative open parenthesis aq closed parenthesis delta upper G degrees = twenty six point three kilojoules per mole
Ca2+(aq) + SO42−(aq) ΔG° = 26.3 kJ/mol upper C a upper S upper O sub 4 open parenthesis s closed parenthesis rightwards harpoon over leftwards harpoon upper C a sup 2 plus open parenthesis aq closed parenthesis plus upper S upper O sub 4 sup 2 negative open parenthesis aq closed parenthesis delta upper G degrees = twenty six point three kilojoules per mole
The standard free energy change for the dissociation of CaSO4upper C a upper S upper O sub 4 in water at 25.0°C is shown above. Given this information, what is the value of the equilibrium constant (Ksp)open parenthesis upper K sub s p closed parenthesis for this reaction?
- 3.49 × 10−1three point four nine times ten to the minus one
- 4.24 × 10−2four point two four times ten to the minus two
- 2.44 × 10−5two point four four times ten to the minus five
- 2.40 × 10−11two point four zero times ten to the minus eleven
Answer to question 35
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0017) This question can be solved using the equation ΔG° = −RTlnK or the equation ΔG° = −2.303RTlogK. The values of ΔG° (26.3 kJ/mol) and T (298 K) are given and the ideal gas constant, R, is 8.31 J/mol∙K. By substituting these values into one of the equations and solving for K, the equilibrium constant for the dissociation of CaSO4 (Ksp) is determined to be 2.44 × 10−5. This Ksp value indicates that the reaction favors the reactant over the products. This is consistent with the positive value of ΔG° and the known relative insolubility of CaSO4 in water.
Correct Response: C. This question can be solved using the equation delta G degrees = negative RTlnK or the equation delta G degrees = negative two point three zero three RT log K. The values of delta G degrees open parenthesis twenty six point three kilojoules per mole closed parenthesis and T open parenthesis two hundred ninety eight K closed parenthesis are given and the ideal gas constant, R, is eight point three one joules per mole bullet operator K. By substituting these values into one of the equations and solving for K, the equilibrium constant for the dissociation of upper C a upper S upper O sub 4 open parenthesis K sub s sub p closed parenthesis is determined to be two point four four times ten to the minus five. This K sub s sub p value indicates that the reaction favors the reactant over the products. This is consistent with the positive value of delta G degrees and the known relative insolubility of upper C a upper S upper O sub 4 in water.
Question 36
36. Use the equation below to answer the question that follows.
C(s) + CO2(g) ↔ 2CO(g)capital C solid plus capital C O subscript 2 gas is in equilibrium with 2 capital C O gas
When the system shown in the equation reaches equilibrium, which of the following expressions best describes the relationship between the concentrations of the species involved in the reaction?
- KP = 2[CO] [C] [CO2] K subscript P equals 2 open bracket capital C O close bracket times open bracket capital C close bracket times open bracket capital C O subscript 2 close bracket
- KP = [CO]2 / [C] [CO2] K subscript P equals open bracket capital C O close bracket superscript 2 divided by open bracket capital C close bracket times open bracket capital C O subscript 2 close bracket
- KP = [C] [CO]2 /2[CO2] K subscript P equals open bracket capital C close bracket times open bracket capital C O close bracket superscript 2 divided by 2 open bracket capital C O subscript 2 close bracket
- KP = [CO]2 /[CO2] K subscript P equals open bracket capital C O close bracket superscript 2 divided by open bracket capital C O subscript 2 close bracket
Answer to question 36
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0017) The equilibrium constant expression is written by dividing the arithmetic product of the concentration of the reaction products by those of the reactants, with each concentration term raised to the power of its stoichiometric coefficient. Concentrations of pure liquids and solids are not included in the equilibrium constant expression because they are constants.
Correct Response:D. (Objective 0017) The equilibrium constant expression is written by dividing the arithmetic product of the concentration of the reaction products by those of the reactants, with each concentration term raised to the power of its stoichiometric coefficient. Concentrations of pure liquids and solids are not included in the equilibrium constant expression because they are constants.
Question 37
37.
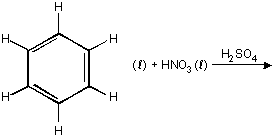
One of the reactants is a liquid aromatic hydrocarbon consisting of six capital C atoms, six capital H atoms, and three double bonds. The six C atoms are bonded to each other in a six-sided ring structure. Each C atom has one H atom bonded to it. The double bonds in this compound are located between the C atoms in positions two and three, four and five and six and one. This aromatic hydrocarbon is reacted with the liquid compound capital H capital N capital O subscript three. The reaction takes place in the presence of the compound capital H subscript two capital S capital O subscript four.
Which of the following is a product of the reaction shown above?
-
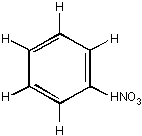 An aromatic hydrocarbon is shown. It consists of six capital C atoms, five capital H atoms, and the substituent, capital H capital N capital O subscript three. The six C atoms are arranged in a six-sided ring structure. Double bonds are present between C atoms in positions two and three, four and five, and six and one. The ring arrangement of the C atoms and the location of the double bonds between C atoms are the same as those of the aromatic hydrocarbon reactant. Five of the C atoms have H atoms bonded to them. The C atom in position three is bonded to the H atom of the substituent H N O subscript three.
An aromatic hydrocarbon is shown. It consists of six capital C atoms, five capital H atoms, and the substituent, capital H capital N capital O subscript three. The six C atoms are arranged in a six-sided ring structure. Double bonds are present between C atoms in positions two and three, four and five, and six and one. The ring arrangement of the C atoms and the location of the double bonds between C atoms are the same as those of the aromatic hydrocarbon reactant. Five of the C atoms have H atoms bonded to them. The C atom in position three is bonded to the H atom of the substituent H N O subscript three.
-
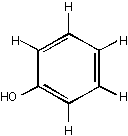 An aromatic hydrocarbon is shown. It consists of six capital C atoms, five capital H atoms, and the substituent, capital O capital H. The six C atoms are arranged in a six-sided ring structure. Double bonds are present between C atoms in positions two and three, four and five, and six and one. The ring arrangement of the C atoms and the location of the double bonds between C atoms are the same as those of the aromatic hydrocarbon reactant. Five of the C atoms have H atoms bonded to them. The C atom in position five is bonded to the O atom of the substituent O H.
An aromatic hydrocarbon is shown. It consists of six capital C atoms, five capital H atoms, and the substituent, capital O capital H. The six C atoms are arranged in a six-sided ring structure. Double bonds are present between C atoms in positions two and three, four and five, and six and one. The ring arrangement of the C atoms and the location of the double bonds between C atoms are the same as those of the aromatic hydrocarbon reactant. Five of the C atoms have H atoms bonded to them. The C atom in position five is bonded to the O atom of the substituent O H.
-
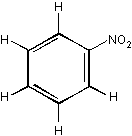 An aromatic hydrocarbon is shown. It consists of six capital C atoms, five capital H atoms, and the substituent, capital N capital O subscript two. The six C atoms are arranged in a six-sided ring structure. Double bonds are present between C atoms in positions two and three, four and five, and six and one. The ring arrangement of the C atoms and the location of the double bonds between C atoms are the same as those of the aromatic hydrocarbon reactant. Five of the C atoms are bonded to H atoms. The C atom in position two is bonded to the N atom of the substituent N O subscript two.
An aromatic hydrocarbon is shown. It consists of six capital C atoms, five capital H atoms, and the substituent, capital N capital O subscript two. The six C atoms are arranged in a six-sided ring structure. Double bonds are present between C atoms in positions two and three, four and five, and six and one. The ring arrangement of the C atoms and the location of the double bonds between C atoms are the same as those of the aromatic hydrocarbon reactant. Five of the C atoms are bonded to H atoms. The C atom in position two is bonded to the N atom of the substituent N O subscript two.
-
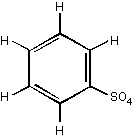 An aromatic hydrocarbon is shown. It consists of six capital C atoms, five capital H atoms, and the substituent, capital S capital O subscript four. The six C atoms are arranged in a six-sided ring structure. Double bonds are present between C atoms in positions two and three, four and five, and six and one. The ring arrangement of the C atoms and the location of the double bonds between C atoms are the same as those of the aromatic hydrocarbon reactant. Five of the C atoms are bonded to H atoms. The C atom in position three is bonded to the S atom of the substituent S O subscript four.
An aromatic hydrocarbon is shown. It consists of six capital C atoms, five capital H atoms, and the substituent, capital S capital O subscript four. The six C atoms are arranged in a six-sided ring structure. Double bonds are present between C atoms in positions two and three, four and five, and six and one. The ring arrangement of the C atoms and the location of the double bonds between C atoms are the same as those of the aromatic hydrocarbon reactant. Five of the C atoms are bonded to H atoms. The C atom in position three is bonded to the S atom of the substituent S O subscript four.
Answer to question 37
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0018) The organic reaction shown above is a type of electrophilic aromatic substitution. Benzene is an aromatic hydrocarbon that is particularly stable as a result of electron delocalization around the ring structure. This stability means that benzene typically undergoes substitution (not addition reactions) in which hydrogen atoms in the ring are replaced with other types of atoms. In this case, a nitronium ion is formed when the sulfuric acid donates a proton to the nitric acid. The nitronium ion behaves as an electrophile, breaking a bond between carbon and hydrogen in the benzene ring and forming a new bond with carbon. Thus, the overall reaction is one in which benzene reacts with nitric acid in the presence of sulfuric acid to form nitrobenzene.
Correct Response: C. (Objective 0018) The organic reaction shown above is a type of electrophilic aromatic substitution. Benzene is an aromatic hydrocarbon that is particularly stable as a result of electron delocalization around the ring structure. This stability means that benzene typically undergoes substitution (not addition reactions) in which hydrogen atoms in the ring are replaced with other types of atoms. In this case, a nitronium ion is formed when the sulfuric acid donates a proton to the nitric acid. The nitronium ion behaves as an electrophile, breaking a bond between carbon and hydrogen in the benzene ring and forming a new bond with carbon. Thus, the overall reaction is one in which benzene reacts with nitric acid in the presence of sulfuric acid to form nitrobenzene.
Question 38
38.
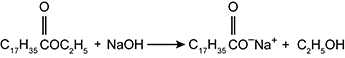
The first reactant is capital C subscript 17 capital H subscript 35, bonded to capital C double bonded to capital O. That same capital C is also bonded to capital O capital C subscript 2 capital H subscript 5. The second reactant is capital N lowercase A capital O capital H. These two reactants combine to yield capital C subscript 2 capital H subscript 5 capital O H plus capital C subscript 17 capital H subscript 35, bonded to capital C double bonded to capital O. That same capital C is also bonded to capital O negative capital N lowercase A positive.
The alkaline hydrolysis of an ester is shown above. This reaction is an example of which of the following types of reactions?
- addition
- condensation
- saponification
- combustion
Answer to question 38
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: C. (Objective 0018) The hydrolysis of an ester in the presence of a base is known as saponification. This is the type of reaction used to make soap.
Correct Response: C. (Objective 0018) The hydrolysis of an ester in the presence of a base is known as saponification. This is the type of reaction used to make soap.
Question 39
39.
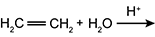
The first reactant is capital H subscript 2 capital C, double bonded to capital C capital H subscript 2. The second reactant is capital H subscript 2 capital O. In the presence of a capital H positive ion, this reaction yields an unrepresented and unknown product.
An alkene is reacting with water in the presence of a strong acid in the chemical reaction shown above. This type of hydration reaction is used to prepare which of the following types of organic compounds?
- amine
- ether
- ketone
- alcohol
Answer to question 39
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: D. (Objective 0018) In the reaction shown, water reacts with ethene (or ethylene) in the presence of a strong acid to produce ethanol. This type of reaction is used in the production of various alcohols.
Correct Response: D. (Objective 0018) In the reaction shown, water reacts with ethene (or ethylene) in the presence of a strong acid to produce ethanol. This type of reaction is used in the production of various alcohols.
Question 40
40. A chemist suspects that an unexpected by-product produced during an organic synthesis reaction contains an amine functional group. Which of the following methods will help the chemist analyze the types of functional groups present in this compound?
- infrared spectroscopy
- column chromatography
- ultracentrifugation
- gel electrophoresis
Answer to question 40
- Answer Enter to expand or collapse answer. Answer expanded
- Correct Response: A. (Objective 0018) When molecules containing covalent bonds absorb infrared radiation, there is an increase in the vibrational motion of their chemical bonds. This change can be detected using infrared spectroscopy. Infrared spectroscopy can be used to determine the types of bonds and functional groups present in a compound. This is possible because different types of bonds will absorb infrared radiation at different wavelengths. Scientists have put together tables correlating specific types of chemical bonds and functional groups with absorption at specific wavelengths. This information can be used to analyze an unknown compound's infrared spectrum and identify the types of functional groups present.
Correct Response: A. (Objective 0018) When molecules containing covalent bonds absorb infrared radiation, there is an increase in the vibrational motion of their chemical bonds. This change can be detected using infrared spectroscopy. Infrared spectroscopy can be used to determine the types of bonds and functional groups present in a compound. This is possible because different types of bonds will absorb infrared radiation at different wavelengths. Scientists have put together tables correlating specific types of chemical bonds and functional groups with absorption at specific wavelengths. This information can be used to analyze an unknown compound's infrared spectrum and identify the types of functional groups present.